Annotate-a-genome
Jump to navigation
Jump to search
Project Goals
- Annotate and add newly sequenced Borrelia genomes to BorreliaBase
- Build an informatics pipeline for gene prediction, ortholog calls, databasing, and synteny analysis
Claim your assigned genome
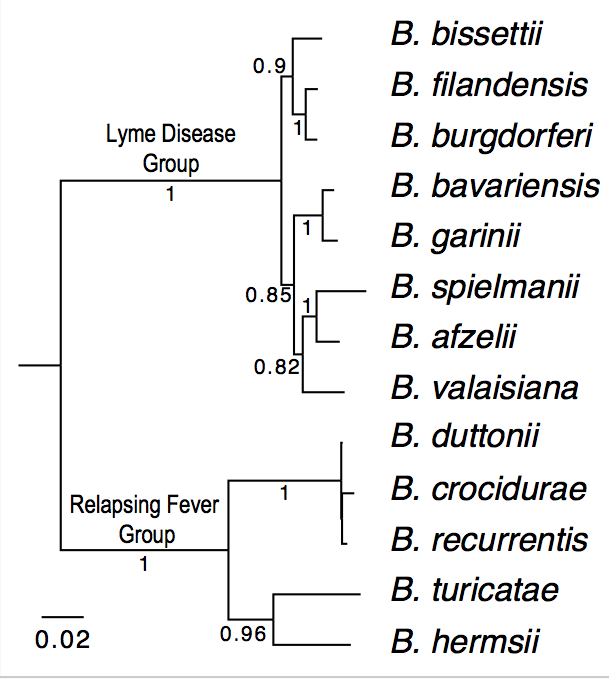
| Genome_id | Strain | Species | Group | Genome Sequences | Notes |
|---|---|---|---|---|---|
| 100 | B31 | B. burgdorferi (reference genome) | Lyme Disease | Reference. Already downloaded as "ref.pep" | |
| 114 | CA382 | B. burgdorferi (California) | Lyme Disease |
| |
| 115 | CA8 | B. burgdorferi (California) | Lyme Disease |
| |
| 304 | BgVir | B. garinii (Russia) | Lyme Disease |
| |
| 305 | NMJW1 | B. garinii (China) | Lyme Disease |
| |
| 402 | HLJ01 | B. afzelii (China) | Lyme Disease |
| |
| 1003 | Ly | B. duttonii (Tanzania) | Relapsing Fever |
|
|
| 1001 | A1 | B. recurrentis (Ethiopia) | Relapsing Fever |
| |
| 1100 | DAH | B. hermsii (Washington State) | Relapsing Fever |
| |
| 1200 | 91E135 | B. turicatae (Texas) | Relapsing Fever |
| |
| 1002 | Achema | B. crocidurae (Mauritania) | Relapsing Fever |
|
|
| 1400 | HR1 | B. parkeri (??) | Relapsing Fever |
| |
| 1300 | LB-2001 | B. miyamotoi (Northeast US) | Relapsing Fever |
| |
| 107 | 94a | B. burgdorferi (Northeast US) | Lyme Disease |
|
Protocol
Dependencies
- BASH (default shell of Linux OS and Apple OS X)
- Perl and BioPerl
- DNATweezer
- NCBI Standalone BLAST+
Part 1. Fetch genome sequences and extract protein sequences
- Note: These scripts are in "../../bio425/annotate-a-genome-pipeline". You may either make a copy to your home directory (recommended) or run directly from that directory by including the path
- Commands:
./fetch-genome.pl <your_assigned_accession> # Expected output: "accession.gb"
./gb2pep.pl <accession.gb> # Expected output: "accession.pep"
Part 2. Predict orthologs with reciprocal BLAST
- Note 1: Replace the "accession" in the following commands with your assigned accession number.
- Note 2: You may have to lower the identity (-d, default 90) and length coverage (-l, default 90) for replapsing fever genomes
- Commands:
cp ../../bio425/annotate-genome-pipeline/b31.pep . # get reference genome
makeblastdb -in b31.pep -dbtype 'prot' -parse_seqids -out ref # Prepare the ref genome DB
makeblastdb -in accession.pep -dbtype 'prot' -parse_seqids -out mygenome # Prepare the new genome DB
blastp -query accession.pep -db ref -outfmt '6 qseqid sseqid pident length qlen evalue' -evalue 1e-3 -out accession.fwd # Forward BLAST # customized outfmt 6
blastp -query b31.pep -db mygenome -outfmt '6 qseqid sseqid pident length qlen evalue' -evalue 1e-3 -out accession.rev # Reverse BLAST
./find-reciprocal.pl <accession.fwd> <accession.rev> > accession.orthlogs 2> accession.not-orthologs # Identify orthologs.
./find-reciprocal.pl -d 80 <accession.fwd> <accession.rev> > accession.orthlogs 2> accession.not-orthologs # for replasping-fever genomes
Part 3. Generate database tables & deposit your results
- Note: Use your assigned genome_id in the table above as the argument for the "-g" option
./gb2table.pl -g <genome_id> -c <accession.gb> # Expected output: "accession.contig.txt"
./gb2table.pl -g <genome_id> -f <accession.gb> # Expected output: "accession.orf.txt"
# Check your outputs
wc <accession.contig.txt>
head <accession.contig.txt>
tail <accession.contig.txt>
wc <accession.orf.txt>
head <accession.orf.txt>
tail <accession.orf.txt>
cp accession.contig.txt ../../bio425/annotate-a-genome-results/your_initial.accession.contig.txt
cp accession.orf.txt ../../bio425/annotate-a-genome-results/your_initial.accession.orf.txt
cp accession.orthologs ../../bio425/annotate-a-genome-results/your_initial.accession.orth
cp accession.not-orthologs ../../bio425/annotate-a-genome-results/your_initial.accession.not-orth