Biol375 2014: Difference between revisions
imported>Weigang |
imported>Weigang |
||
| (26 intermediate revisions by the same user not shown) | |||
| Line 126: | Line 126: | ||
|- style="background-color:lightsteelblue;" | |- style="background-color:lightsteelblue;" | ||
! Assignment #8 (5 pts; Due 11/10) | ! Assignment #8 (5 pts; Due 11/10) | ||
|- style="background-color: | |- style="background-color:white;" | ||
| | | An international team of scientists recently sequenced 99 genomes of ebola viruses. They reported their work in [http://www.sciencemag.org/content/345/6202/1369.full?sid=6b4ac53f-18af-4b71-8f41-87e3a51c2105 this recent publication]. | ||
# Go to the the [http://phylogeny.lirmm.fr/phylo_cgi/index.cgi phylogeny.fr website] and select "Phylogenetic Analysis" and then "One Click" analysis | # Go to the the [http://phylogeny.lirmm.fr/phylo_cgi/index.cgi phylogeny.fr website] and select "Phylogenetic Analysis" and then "One Click" analysis | ||
# Copy and paste | # Copy and paste [[Datafile|these VP30 sequences]] into the text box and click on "Submit" | ||
# When analysis is finished, you should see a phylogenetic tree. Re-root the tree using three strains isolated on 1976 or 1977 as outgroup. Save and print the tree. Answer the following questions with explanation. | # When analysis is finished, you should see a phylogenetic tree. Re-root the tree using three strains isolated on 1976 or 1977 as outgroup. Save and print the tree. Answer the following questions with explanation. | ||
## | ## Name the alignment program and the phylogenetic methods (Distance, parsimony, likelihood, or other method?) used to produce your tree | ||
## Are isolates collected from different years all monophylogenetic, all paraphyletic, or some monophyletic and some paraphyletic? | ## Are isolates collected from different years all monophylogenetic, all paraphyletic, or some monophyletic and some paraphyletic? | ||
## Are outbreaks in different years independent from each other, or one outbreak leads to another? | ## Are outbreaks in different years independent from each other, or one outbreak leads to another? | ||
## What would you conclude based on your tree regarding the reservoir source of the ebolavirus: Are Ebolaviruses more likely to have a human or non-human reservoir? | ## What would you conclude based on your tree regarding the reservoir source of the ebolavirus: Are Ebolaviruses more likely to have a human or non-human reservoir? | ||
|} | |} | ||
* 11/6 (TH). Distance methods (Chapter 6) | * 11/6 (TH). Distance methods (Chapter 6). | ||
* 11/10 (M). Likelihood methods (Chapter 6) | * 11/10 (M). Likelihood methods (Chapter 6) | ||
* 11/13 (TH). Tree-testing (Chapter 6) | {| class="wikitable sortable mw-collapsible" | ||
|- style="background-color:lightsteelblue;" | |||
! Assignment #9 (5 pts; Due 11/13, Thursday) | |||
|- style="background-color:white;" | |||
| Compare [[Datafile|these two Ebola VP30 sequences]], one from the 2014 outbreak and the other from the 1994 outbreak. | |||
# Calculate Jukes-Cantor distance between the two sequences (specify unit) | |||
# Identify the number of transitions and transversions | |||
# Identify the number of synonymous and nonysynonymous substitutions | |||
# Assuming that the total number of synonymous sites S=174 and the total number of nonsynonymous sites N=690, calculate <i>d<sub>s</sub> and d<sub>n</sub></i> (with Jukes-Cantor correction) | |||
|} | |||
* 11/13 (TH). Tree-testing & Review (Chapter 6). Lecture slides: [[File:Part-3-tree-construction-small.pdf|thumbnail]] | |||
* 11/17 (M). '''Midterm Exam 3''' | * 11/17 (M). '''Midterm Exam 3''' | ||
===Part 4. Population Genetics === | ===Part 4. Population Genetics === | ||
* <font color="gray">11/20 (TH). Instructor traveling. No class</font> | * <font color="gray">11/20 (TH). Instructor traveling. No class</font> | ||
* 11/24 (M). | * 11/24 (M). Mechanism of molecular evolution: Overview & SNP statistics | ||
{| class="wikitable sortable mw-collapsible" | |||
|- style="background-color:lightsteelblue;" | |||
! Assignment #10 (10 pts; Due 12/4, Thursday) | |||
|- style="background-color:lightblue;" | |||
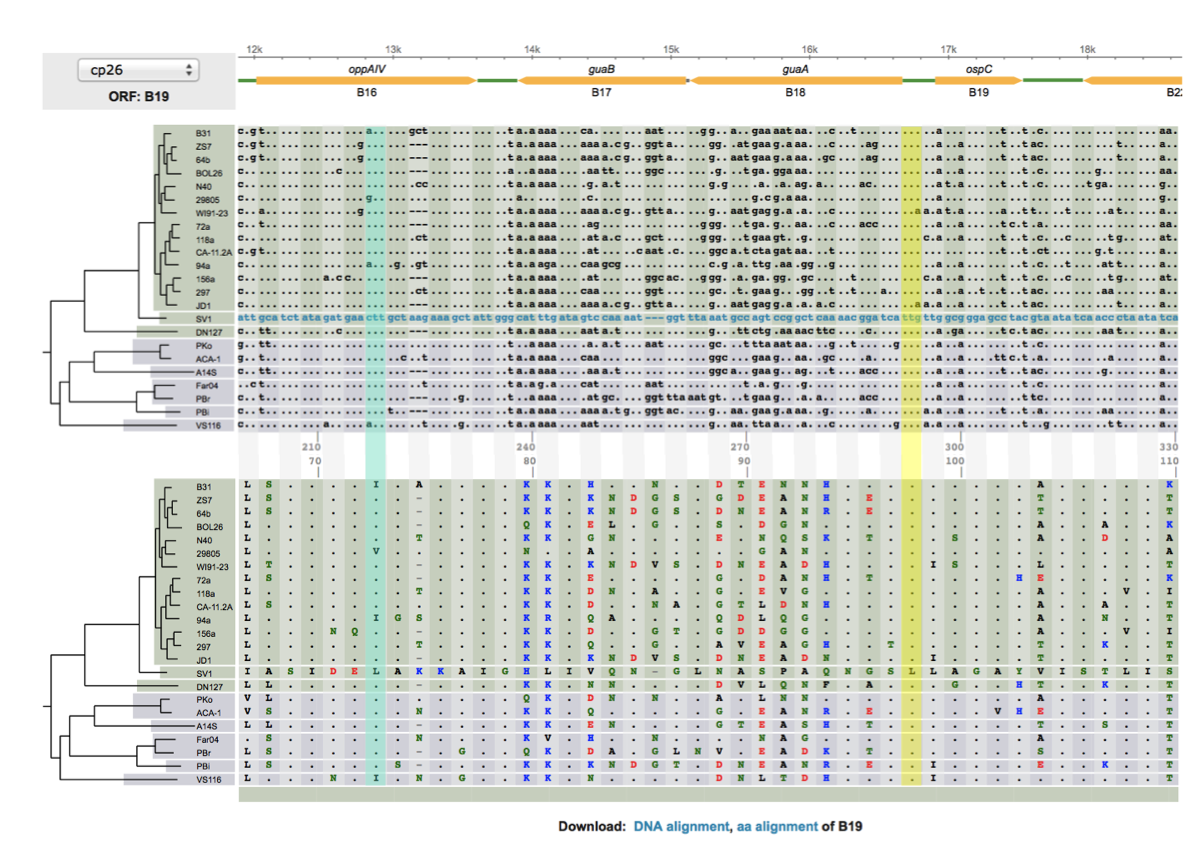
|[[File:Snp-pa1.png|thumbnail]] | |||
The left figure shows a codon alignment of 38 strains of a bacterium, with an outgroup sequence (which starts with a string of SNPs: "....g...c..ca..", etc), answer the following questions (<font color="red">with the outgroup sequence excluded.</font>) <font color="green">Do not print the figure directly. Hand-copy the sequences to a graph sheet, include only sequences at the two variable codon positions</font>: | |||
# There are two SNP sites. For each SNP, determine whether it is a synonymous or nonsynonymous change (could be both if more than 2 states). You may simply list the codons and their corresponding amino acids, at each aligned codon site. | |||
# Calculate allele frequencies at each SNP site (for 3 SNP states, calculate frequencies of all three separately) | |||
# List all haplotypes using the 2 SNP sites | |||
# Calculate frequencies of all haplotypes | |||
# Using the outgroup sequence, determine the ancestral and derived SNP, codon, and amino-acid states at each codon site. Without the outgroup sequence, could derived and ancestral states be determined (e.g., by majority)? Explain with a tree including the outgroup sequence. | |||
# (Bonus: +2) For sites that are fixed differences between the outgroup sequences and others (e.g., the 5th nucleotide site), could one determine which is the ancestral and which is the derived state? Explain with a tree. | |||
|} | |||
* <font color="gray">12/1 (M). Instructor traveling. No class</font> | * <font color="gray">12/1 (M). Instructor traveling. No class</font> | ||
* 12/4 (TH). | * 12/4 (TH). Genetic Drift | ||
* 12/8 (M). | * 12/8 (M). Neutral Theory & Molecular Clock | ||
* 12/11 (TH). | * 12/11 (TH). Tests of Natural Selection. Lecture Slides: [[File:Part-4-evol-mechanisms.pdf|thumbnail]] | ||
* 12/15 (M). Review | * 12/15 (M). Review & Course evaluations. Review slides: [[File:Final-review-slides.pdf|thumbnail]]. '''Submit your Teacher's Evaluation''', using either: | ||
* 12/ | ** Personal computer at [http://www.hunter.cuny.edu/te www.hunter.cuny.edu/te]; or, | ||
** Smartphone at [http://www.hunter.cuny.edu/mobilete www.hunter.cuny.edu/mobilete] | |||
* 12/18 (TH) '''Comprehensive Final Exam''' (Regular class hours & Room) | |||
* 12/31 (Wed). Grades Submitted to Registrar Offices (Hunter and Graduate Center) | * 12/31 (Wed). Grades Submitted to Registrar Offices (Hunter and Graduate Center) | ||
Latest revision as of 01:43, 16 December 2014
Course Description
Molecular evolution is the study of the change of DNA and protein sequences through time. Theories and techniques of molecular evolution are widely used in species classification, biodiversity studies, comparative genomics, and molecular epidemiology. Contents of the course include:
- Population genetics, which is a framework of understanding mechanisms of sequence evolution through mutation, recombination, gene duplication, genetic drift, and natural selection.
- Molecular systematics, which introduces statistical models of sequence evolution and methods of reconstructing species phylogeny.
- Bioinformatics, which provides hands-on training on data acquisition and the use of software tools for phylogenetic analyses.
This 3-credit course is designed for upper-level biology-major undergraduates. Hunter pre-requisites are BIOL203, and MATH150 or STAT113.
Textbooks
- (Required) Roderic M. Page and Edward C. Holmes,1998, Molecular Evolution: A phylogenetic Approach, Blackwell Science Ltd.
- (Recommended) Baum & Smith, 2013. Tree Thinking: an Introduction to Phylogenetic Biology, Roberts & Company Publishers, Inc.
Learning Goals
- Understand mechanisms of DNA sequence evolution
- Be able to describe evolutionary relationships using phylogenetic trees
- Understand the computational algorithms for building phylogenetic trees
- Be able to use web-based as well as stand-alone software to infer phylogenetic trees
Links for phylogenetic tools
Exams & Grading
- Assignments. All assignments should be handed in as hard copies only. Email submission will not be accepted. Late submissions will receive 10% deduction (of the total grade) per day.
- Three Mid-term Exams (30 pts each)
- Comprehensive Final Exam (50 pts)
Bonus for active participation in classroom discussions
Academic Honesty
While students may work in groups and help each other for assignments, duplicated answers in assignments will be flagged and investigated as possible acts of academic dishonesty. To avoid being investigated as such, do NOT copy anyone else's work, or let others copy your work. At the least, rephrase using your own words. Note that the same rule applies regarding the use of textbook and online resources: copied sentences are not acceptable and will be considered plagiarism.
Hunter College regards acts of academic dishonesty (e.g., plagiarism, cheating on examinations, obtaining unfair advantage, and falsification of records and official documents) as serious offenses against the values of intellectual honesty. The College is committed to enforcing the CUNY Policy on Academic Integrity and will pursue cases of academic dishonesty according to the Hunter College Academic Integrity Procedures.
Course Schedule
Part 1. Tree Thinking
- 8/28 (TH). Overview & Introduction. Lecture slides:
| Assignment 1 (10 pts; Due: 9/4, Thursday) |
|---|
- 9/1 (M). Labor Day. No class
- 9/4 (TH). 1.1. Introduction (Continued). In-class exercise 1.
| Assignment 2 (5 pts; Due: 9/8, Monday) |
|---|
Watch Origin of Species: Lizards in an Evolutionary Tree. Provide short answer (1-3 sentences) to each of the following three questions.
|
- 9/8 (M). 2.1. Intro to trees
- 9/11 (TH). 2.2 & 2.3. Tree Distance. In-class exercise 2.
| Assignment 3 (5 pts; Due: 9/15, Monday) |
|---|
| Computer exercise. Obtain an account on EvolView. Once logged in, under the "Basic" tab, click the first icon & copy and paste the following NEWICK string: "(monkey:0.09672,((tarsier:0.18996,lemur:0.14790)0.999:0.09005,(macaque:0.18524,(gibbon:0.10388,(orang-utan:0.09481,(human:0.03391,(gorilla:0.06135,chimpanzee:0.05141):0.01580)0.316:0.05381)1.000:0.03019)0.978:0.05616)0.997:0.05042)0.965:0.09672);". Name your project as "Assignment 3" and the tree as "primate". Render the tree in all five available formats. Using the "Export" tab to download all tree graphs (in "jpeg" or "png" format). Copy and Paste your tree graphs into a single page of Microsoft Word or PowerPoint. Turn in a printed hard copy. |
- 9/15 (M). 2.4 & 2.5. Species Tree & Lineage Sorting
- 9/18 (TH). 2.5. Consensus Tree & Review. Chapter 2 Slides:. In-class Exercise 3:
- 9/22 (M). Midterm Exam I
Part 2. Trait Evolution
- 9/25 (TH). Holiday Recess. No Class
- 9/29 (M). Traits & trait matrix
| Assignment #4 (5 pts; Due 10/6) |
|---|
| Based on the lizard card, construct a character-state matrix for all lizard species. For each species, list its character state for each of the following two characters (as columns): (1) Geographic origin, and (2) Habitat. Re-watch the video may help this assignment. Hint: use Excel & hand in a printout of your Excel sheet. |
- 10/2 (TH). Homoplasy & consistency
- 10/6 (M). Parsimony reconstruction (Chapter 5). In-Class Exercise 4:
| Assignment #5 (5 pts; Due 10/9) |
|---|
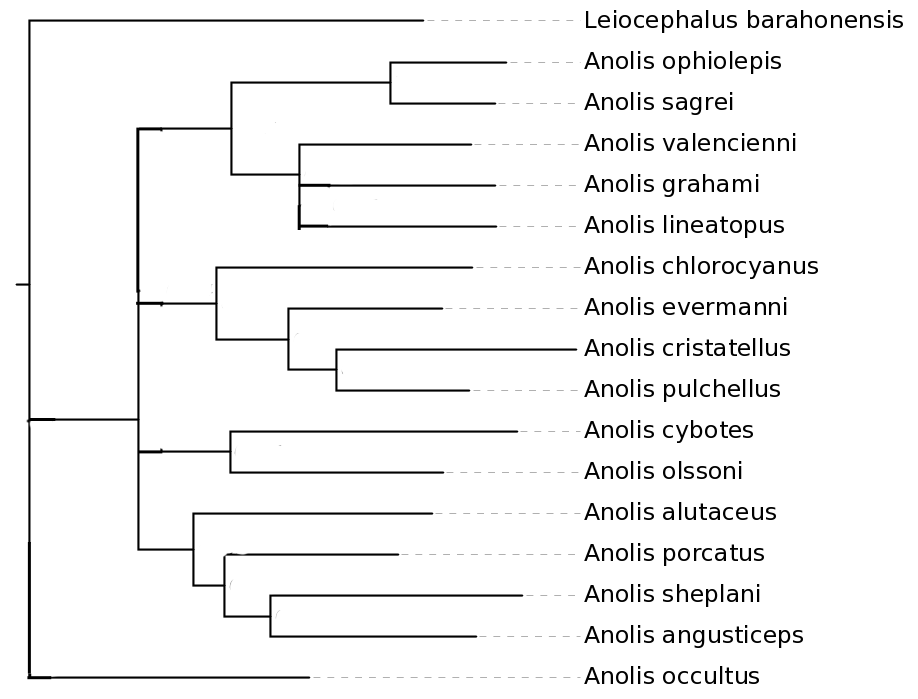
Use EvolView to display the following tree of Caribbean lizards: "((Anolis_chlorocyanus:0.15297,(Anolis_evermanni:0.09207,(Anolis_cristatellus:0.14363,Anolis_pulchellus:0.07962)0.931:0.02884)0.997:0.04280)0.897:0.02232,(Anolis_cybotes:0.17149,Anolis_olssoni:0.12747)0.974:0.03034,(((Anolis_ophiolepis:0.06969,Anolis_sagrei:0.06284)1.000:0.09480,(Anolis_valencienni:0.10249,(Anolis_grahami:0.10016,Anolis_lineatopus:0.10064)0.613:0.01700)0.999:0.04077)0.997:0.04169,((Leiocephalus_barahonensis:0.24783,Anolis_occultus:0.15489)0.978:0.05261,(Anolis_alutaceus:0.14271,(Anolis_porcatus:0.10377,(Anolis_sheplani:0.15083,Anolis_angusticeps:0.12285)0.943:0.02748)0.898:0.01870)0.989:0.03278)0.514:0.01385)0.404:0.01061);" Note:
|
- 10/9 (TH). Genome & gene structure (Chapter 3)
| Assignment #6 (10 pts; Due 10/16) |
|---|
|
- 10/13 (M). No Class
- 10/16 (TH). Genome and gene evolution. Lecture slides (with answer keys to assignments & in-class exercises):
- 10/20 (M). Review & Practices
- 10/23 (TH). Midterm Exam 2
Part 3. Tree Algorithms
- 10/27 (M). BLAST & Alignments (Chapter 5)
| Assignment #7 (5 pts; Due 11/3) |
|---|
Based on the NCBI Gene Page for cytochrome C (CYCS), answer the following questions:
|
- 10/30 (TH). Maximum parsimony (Chapter 6). In class exercise #6:
- 11/3 (M). Genetic distances (Chapter 6)
| Assignment #8 (5 pts; Due 11/10) |
|---|
An international team of scientists recently sequenced 99 genomes of ebola viruses. They reported their work in this recent publication.
|
- 11/6 (TH). Distance methods (Chapter 6).
- 11/10 (M). Likelihood methods (Chapter 6)
| Assignment #9 (5 pts; Due 11/13, Thursday) |
|---|
Compare these two Ebola VP30 sequences, one from the 2014 outbreak and the other from the 1994 outbreak.
|
- 11/13 (TH). Tree-testing & Review (Chapter 6). Lecture slides:
- 11/17 (M). Midterm Exam 3
Part 4. Population Genetics
- 11/20 (TH). Instructor traveling. No class
- 11/24 (M). Mechanism of molecular evolution: Overview & SNP statistics
| Assignment #10 (10 pts; Due 12/4, Thursday) |
|---|
|
The left figure shows a codon alignment of 38 strains of a bacterium, with an outgroup sequence (which starts with a string of SNPs: "....g...c..ca..", etc), answer the following questions (with the outgroup sequence excluded.) Do not print the figure directly. Hand-copy the sequences to a graph sheet, include only sequences at the two variable codon positions:
|
- 12/1 (M). Instructor traveling. No class
- 12/4 (TH). Genetic Drift
- 12/8 (M). Neutral Theory & Molecular Clock
- 12/11 (TH). Tests of Natural Selection. Lecture Slides:
- 12/15 (M). Review & Course evaluations. Review slides: . Submit your Teacher's Evaluation, using either:
- Personal computer at www.hunter.cuny.edu/te; or,
- Smartphone at www.hunter.cuny.edu/mobilete
- 12/18 (TH) Comprehensive Final Exam (Regular class hours & Room)
- 12/31 (Wed). Grades Submitted to Registrar Offices (Hunter and Graduate Center)