Pseudomonas population genomics: Difference between revisions
Jump to navigation
Jump to search
imported>Rayrah |
imported>Rli0019 mNo edit summary |
||
| (11 intermediate revisions by 2 users not shown) | |||
| Line 12: | Line 12: | ||
## Reconstruct phylogenetic tree | ## Reconstruct phylogenetic tree | ||
## Run PAML tests | ## Run PAML tests | ||
==Update:August 5, 2013== | |||
Pseudomonas Pipeline Steps: | |||
USAGE: ./pseudo_pipline.sh [options] [FASTQ file] | |||
*'''Step 0''': Creation of Simulated reads via GemSim: http://www.biomedcentral.com/1471-2164/13/74 (outputs FastQ file with quality scores). | |||
*'''Step 1''': Assembly of reads via ALL Paths | |||
*'''Step 2''': ORF finding via Glimmer | |||
*'''Step 3''': Gene finding via BLAST | |||
*'''Step 4''': Top Gene matches and Database orth_id matching via top_orth_match.pl | |||
*'''Step 5''': Predicted Ortholog Database import | |||
*'''Step 6''': Core genome extraction | |||
*'''Step 7''': Choose random number of orthologs via random_cds.sh | |||
*'''Step 8''': Translation of Orthologs via BioSeq | |||
*'''Step 9''': Alignment of peptide files via Muscle | |||
*'''Step 10''': Reverse translation of aligned sequences via align_coding_seq.pl | |||
*'''Step 11''': Creation of Aligned Fasta file via AlignConcat_Bioperl.final.pl | |||
*'''Step 12''': Creation of treefile via FASTTREE | |||
Pseudomonas Pipeline options: | |||
#-i: keep all intermediary files (default remove all intermediate files) | |||
#-f: keep only intermediate fasta files | |||
#-b: [integer] blast score threshold | |||
#-n: [integer] number of genes to create treefile from | |||
==Update:July 22, 2013== | ==Update:July 22, 2013== | ||
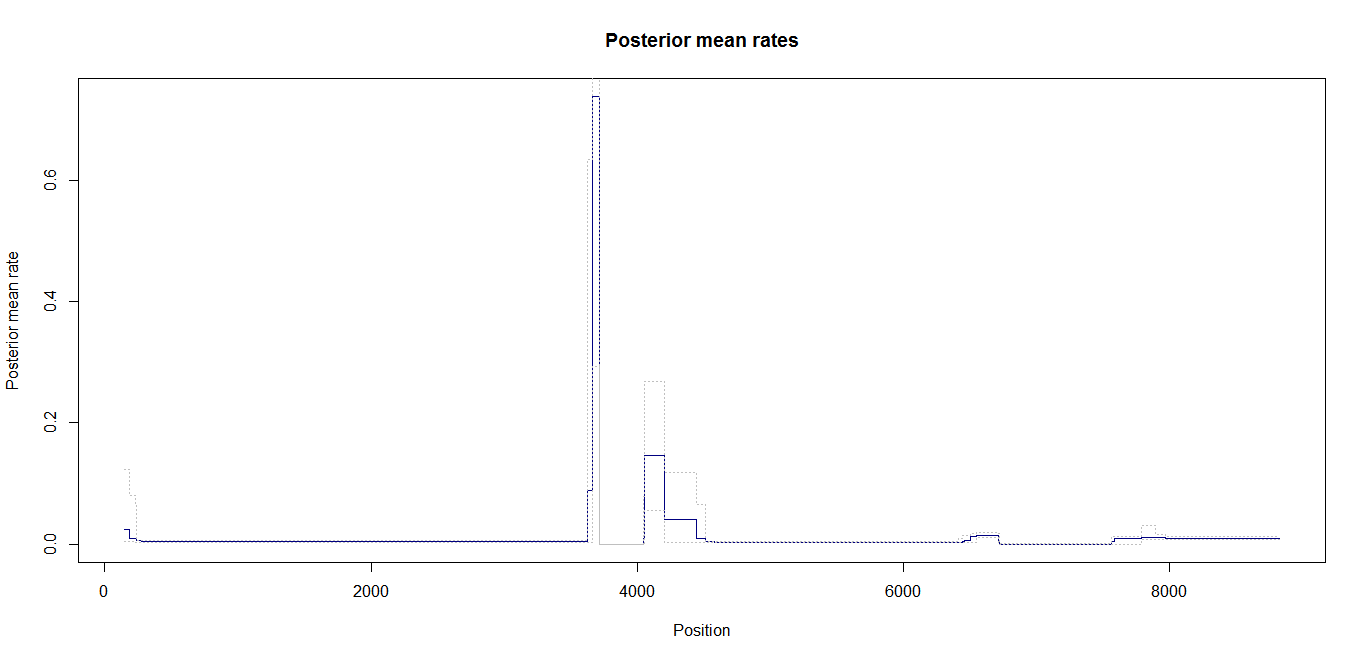
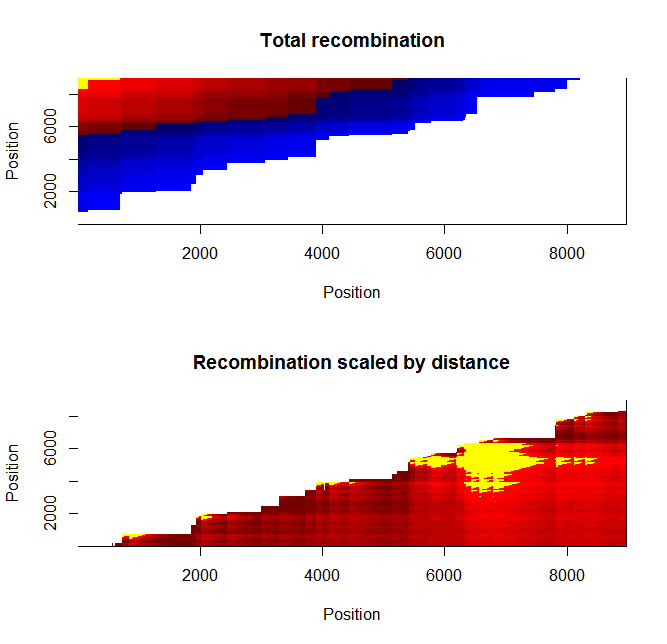
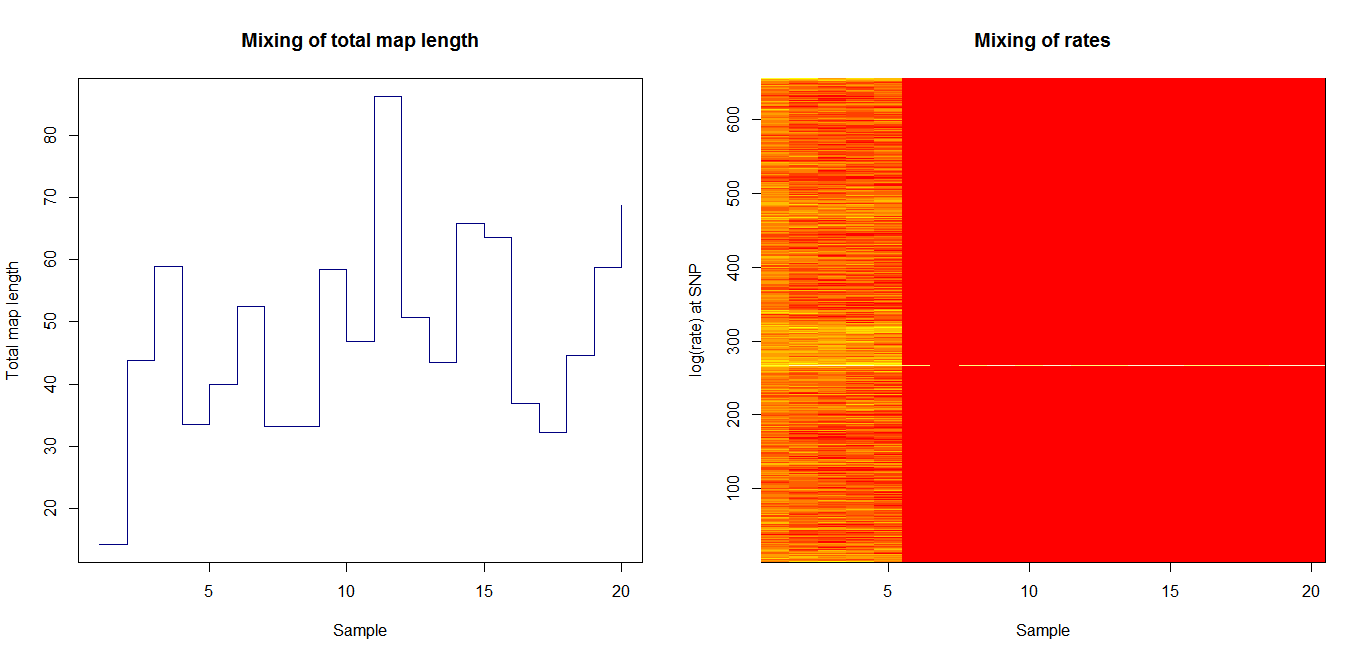
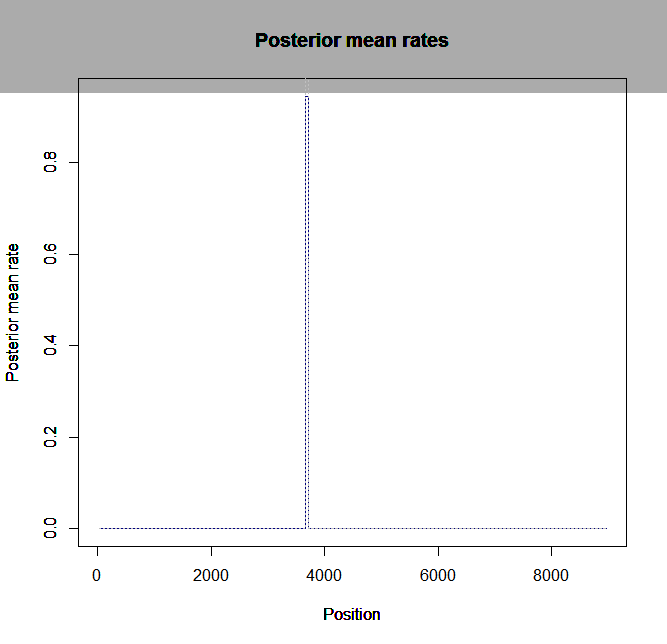
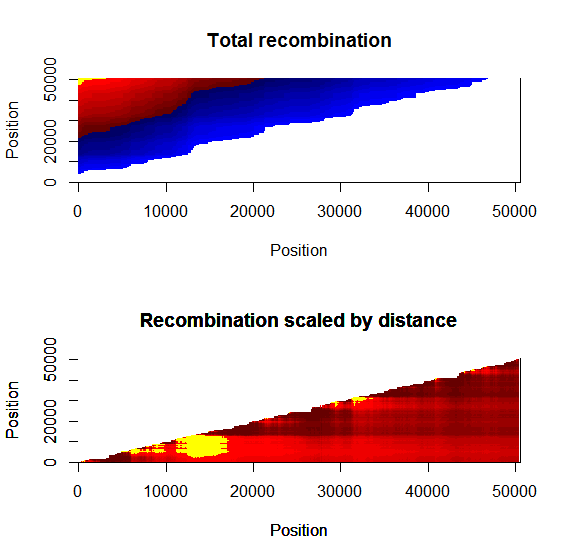
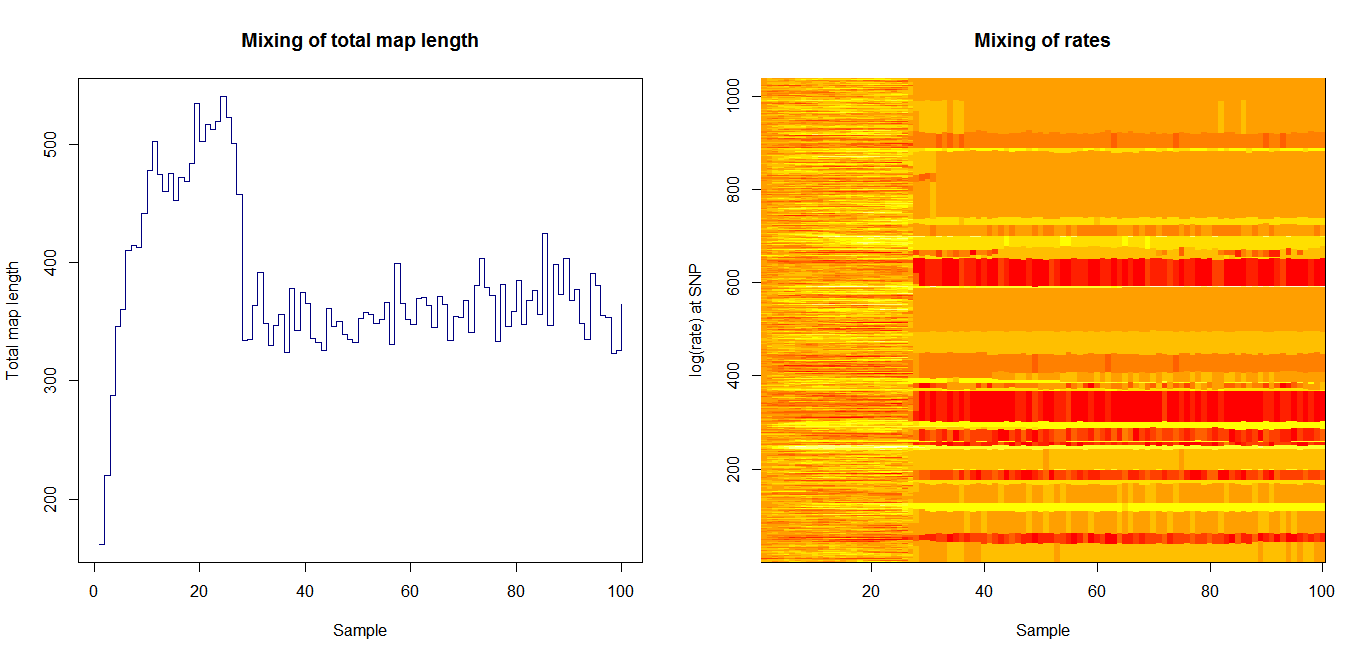
Ld_hat results | Estimation of recombination hotspots via LD_hat | ||
Ld_hat results: | |||
{| class="wikitable" | {| class="wikitable" | ||
| Line 23: | Line 51: | ||
| PA7 || 10 || [[File:PA7_1.png|200px]] || [[File:PA7_2.png|200px]] || [[File:PA7_3.png|200px]] || [[File:PA7_4.png|200px]] | | PA7 || 10 || [[File:PA7_1.png|200px]] || [[File:PA7_2.png|200px]] || [[File:PA7_3.png|200px]] || [[File:PA7_4.png|200px]] | ||
|- | |- | ||
| No PA7 || 50 || [[File: | | No PA7 || 50 || [[File:No_PA7_51.png|200px]] || [[File:No_PA7_6.png|200px]] || [[File:No_PA7_7.png|200px]] || [[File:No_PA7_8.png|200px]] | ||
|} | |} | ||
Latest revision as of 23:41, 1 December 2013
Projects
- Build a local genome database
- Database schema:
- "genome": genome_id, strain_name, ncbi_taxid
- "orf": genome_id, locus_tag, start, stop, strand, genome_name, product_name
- "orth_orf": orth_orf_id, locus_name, genome_id, orth_class
- Parsing scripts
- Rayees Parsing code, requires that you remove columns 9-27 using bash command:
cut -c 1-8(I will write a bash script that does this and runs the program) https://www.dropbox.com/s/lpxxbkxeyw7frrn/parser.pl
- Rayees Parsing code, requires that you remove columns 9-27 using bash command:
- Database loading scripts
- Database schema:
- Molecular Evolution of flagellum genes
- Download orthologs
- Reconstruct phylogenetic tree
- Run PAML tests
Update:August 5, 2013
Pseudomonas Pipeline Steps:
USAGE: ./pseudo_pipline.sh [options] [FASTQ file]
- Step 0: Creation of Simulated reads via GemSim: http://www.biomedcentral.com/1471-2164/13/74 (outputs FastQ file with quality scores).
- Step 1: Assembly of reads via ALL Paths
- Step 2: ORF finding via Glimmer
- Step 3: Gene finding via BLAST
- Step 4: Top Gene matches and Database orth_id matching via top_orth_match.pl
- Step 5: Predicted Ortholog Database import
- Step 6: Core genome extraction
- Step 7: Choose random number of orthologs via random_cds.sh
- Step 8: Translation of Orthologs via BioSeq
- Step 9: Alignment of peptide files via Muscle
- Step 10: Reverse translation of aligned sequences via align_coding_seq.pl
- Step 11: Creation of Aligned Fasta file via AlignConcat_Bioperl.final.pl
- Step 12: Creation of treefile via FASTTREE
Pseudomonas Pipeline options:
- -i: keep all intermediary files (default remove all intermediate files)
- -f: keep only intermediate fasta files
- -b: [integer] blast score threshold
- -n: [integer] number of genes to create treefile from
Update:July 22, 2013
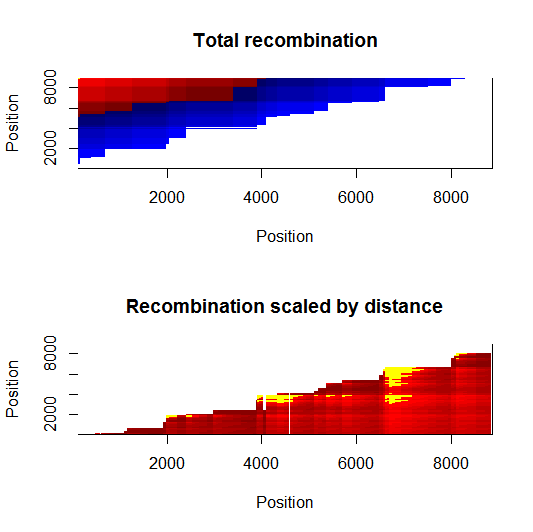
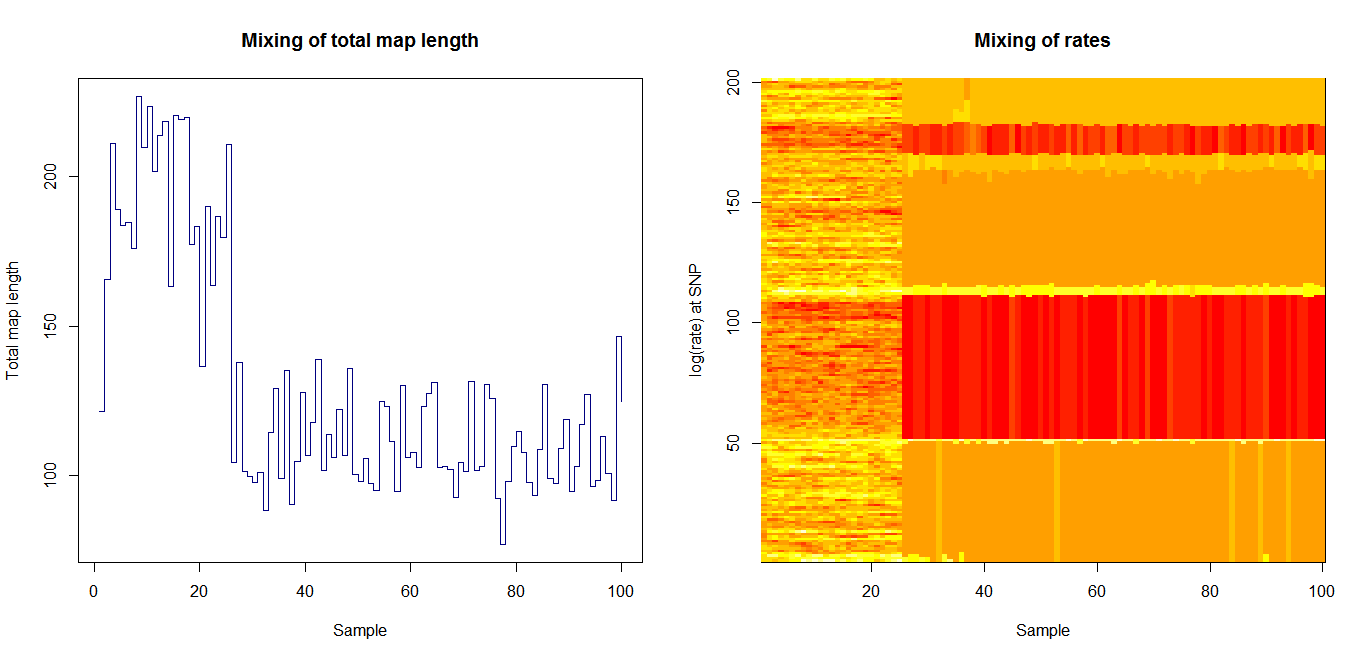
Estimation of recombination hotspots via LD_hat Ld_hat results:
| Data | Number of Genes | ||||
| No PA7 | 10 | 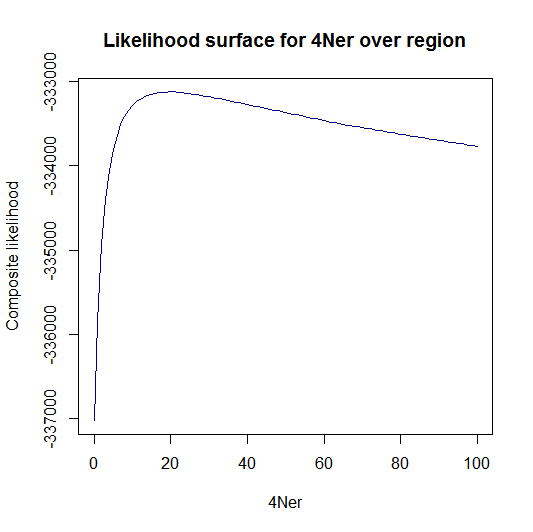 |
 |
 |
|
| PA7 | 10 | 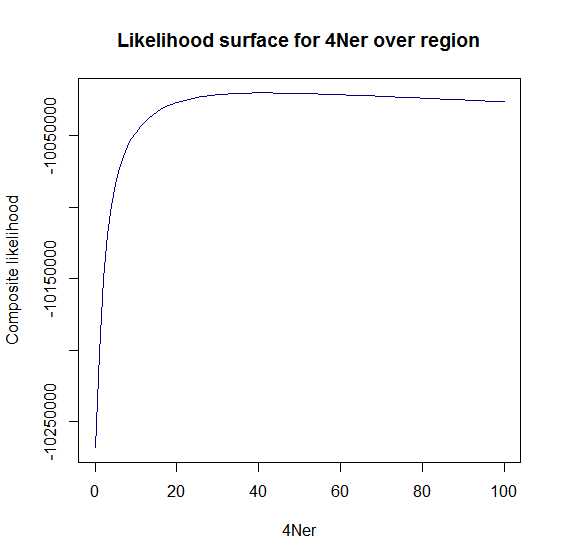 |
 |
 |
|
| No PA7 | 50 | 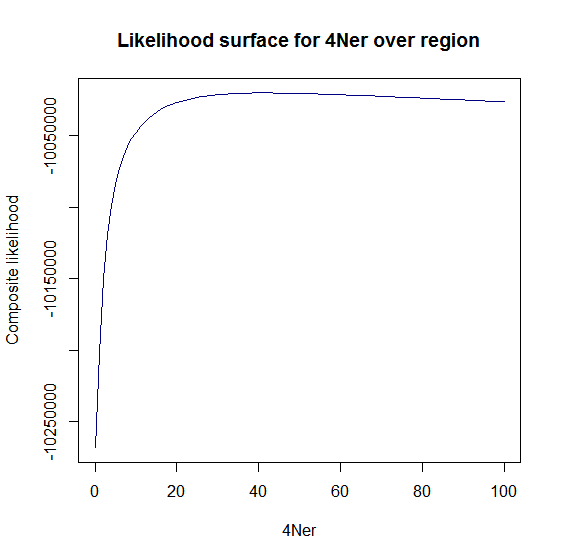 |
 |
 |
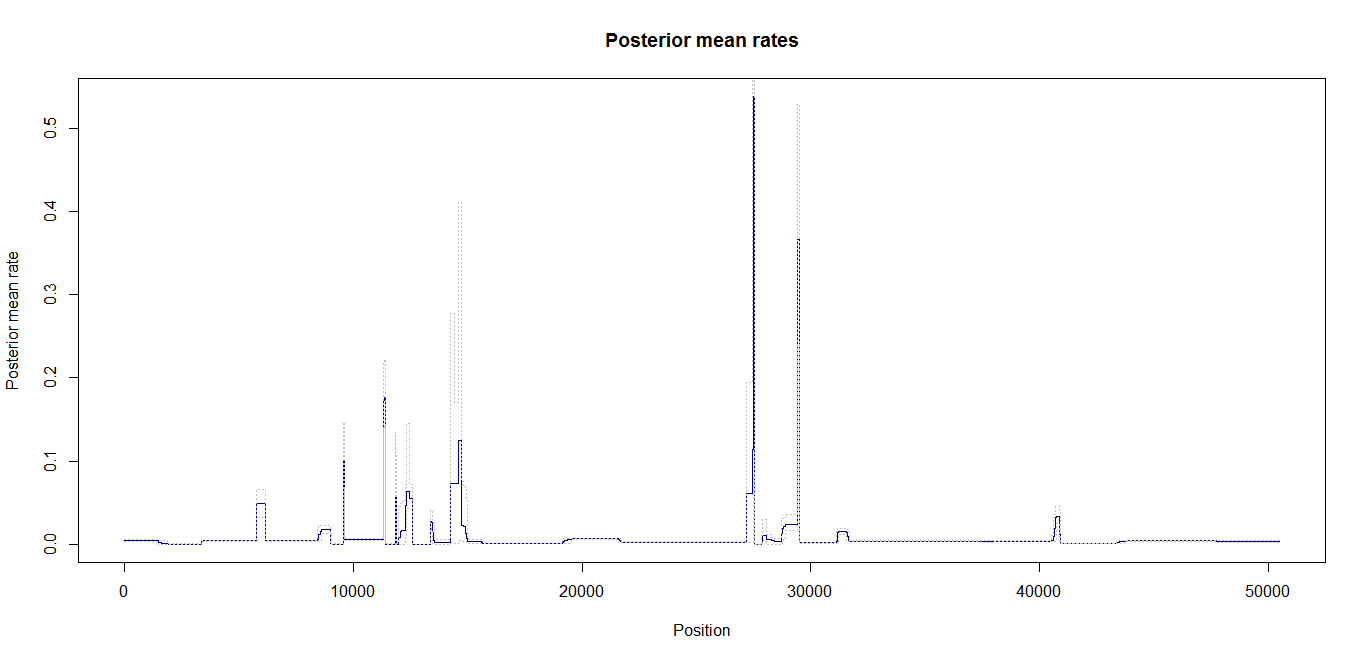
|
Update:July 17, 2013
Phylip dollop parsimony tree based on an matrix of ortholog changes
Update: July 16, 2013
Treefiles based on aligned orthologs
| number of genes used | Method | treefile |
| 100 | FASTTREE | treefile |
| 100 | FASTTREE | Treefile 2 |
| all orthologs | Phylip | treefile_phylip |
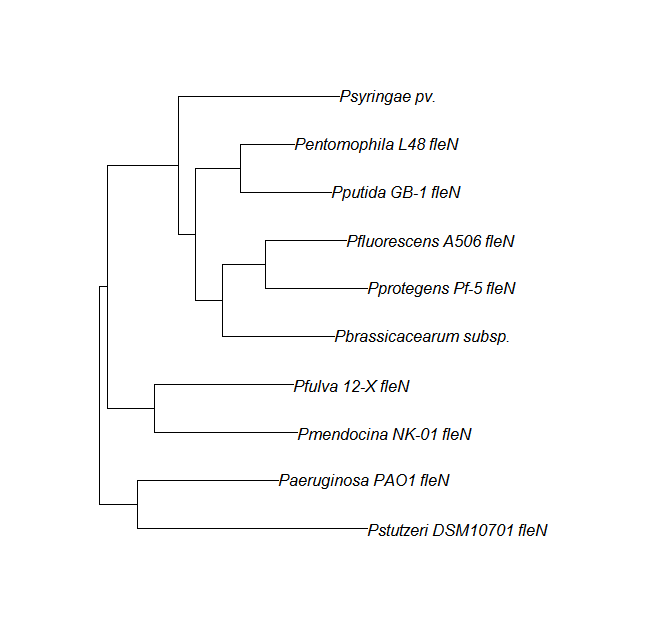
FleN, FleQ, Flhf unique strains
| Gene | alignment | tree |
| fleN | fleN unique align | |
| fleQ | fleQ unique align | 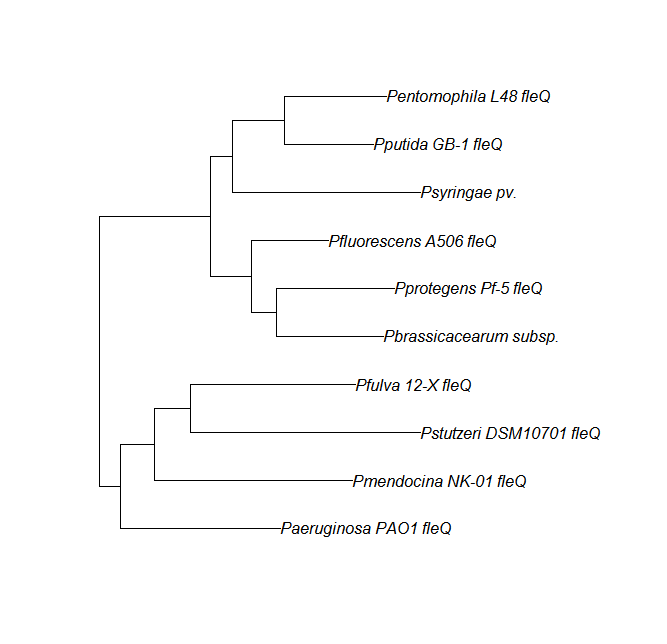
|
| flhf | flhF unique align | 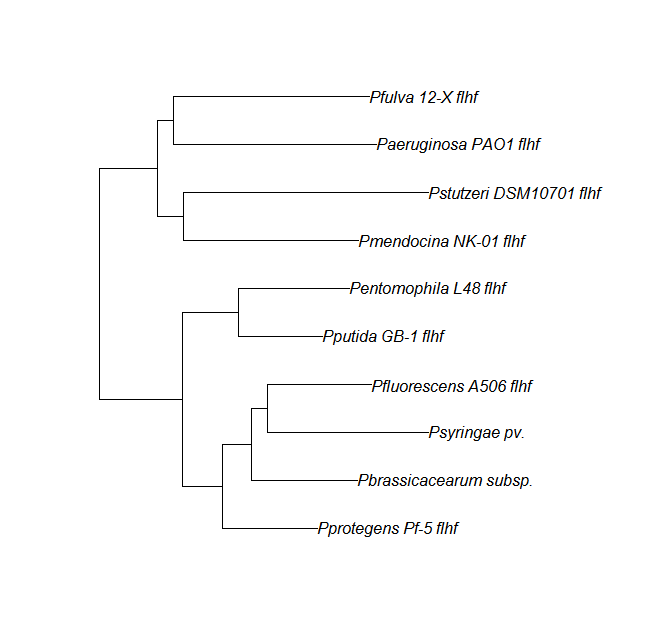
|
To do:
- Create pipeline
- LD_Hat w/ 10 genes excluding and including PA7
Update: June 28, 2013
Phylogenetic Analysis by Maximum Likelihood (PAML) Test performed on FleN, FleQ, FlhF orthologs and aeruginosa only orthologs
| Gene | PAML outfile for orthologs | |
|---|---|---|
| fleN | fleN PAML | |
| fleQ | fleQ PAML | |
| flhf | flhF-corrected PAML |
To do:
- Estimate genome tree using MrBayes and BEST (http://www.stat.osu.edu/~dkp/BEST/introduction/).
- Analysis of positively selected genes using PAML and homozygosity analysis
Update: June 18, 2013
Ortholog aligning and phylogenetic tree material and methods
Protein ortholog data: fleN, fleQ, flhF
- Protocol:
- Fasta headers are too long for tree, run: rename.pl to shorten names. Usage:
>rename.pl <FASTA_file> > <OUTPUTfilename.fas>(will rewrite script to create automatic output file) - To align use muscle Usage:
>muscle -in <FASTA_File> -out <OUTPUTfilename> -clwstrict - To create tree file run clustalW2 Usage
>clustalw2 -infile= <Aligned_file> -output= Phylip - To create tree run R using following commands:
setwd("<directory containing phylip files>")library ("ape")library ("phangorn")<gene_name> = read.tree("<file_gene_name.phy>")<gene_name> = midpoint(gene_name)plot(<gene_name>)
- Fasta headers are too long for tree, run: rename.pl to shorten names. Usage:
| Gene | Alignment | Tree | Notes |
|---|---|---|---|
| fleN | fleN pep alignment | 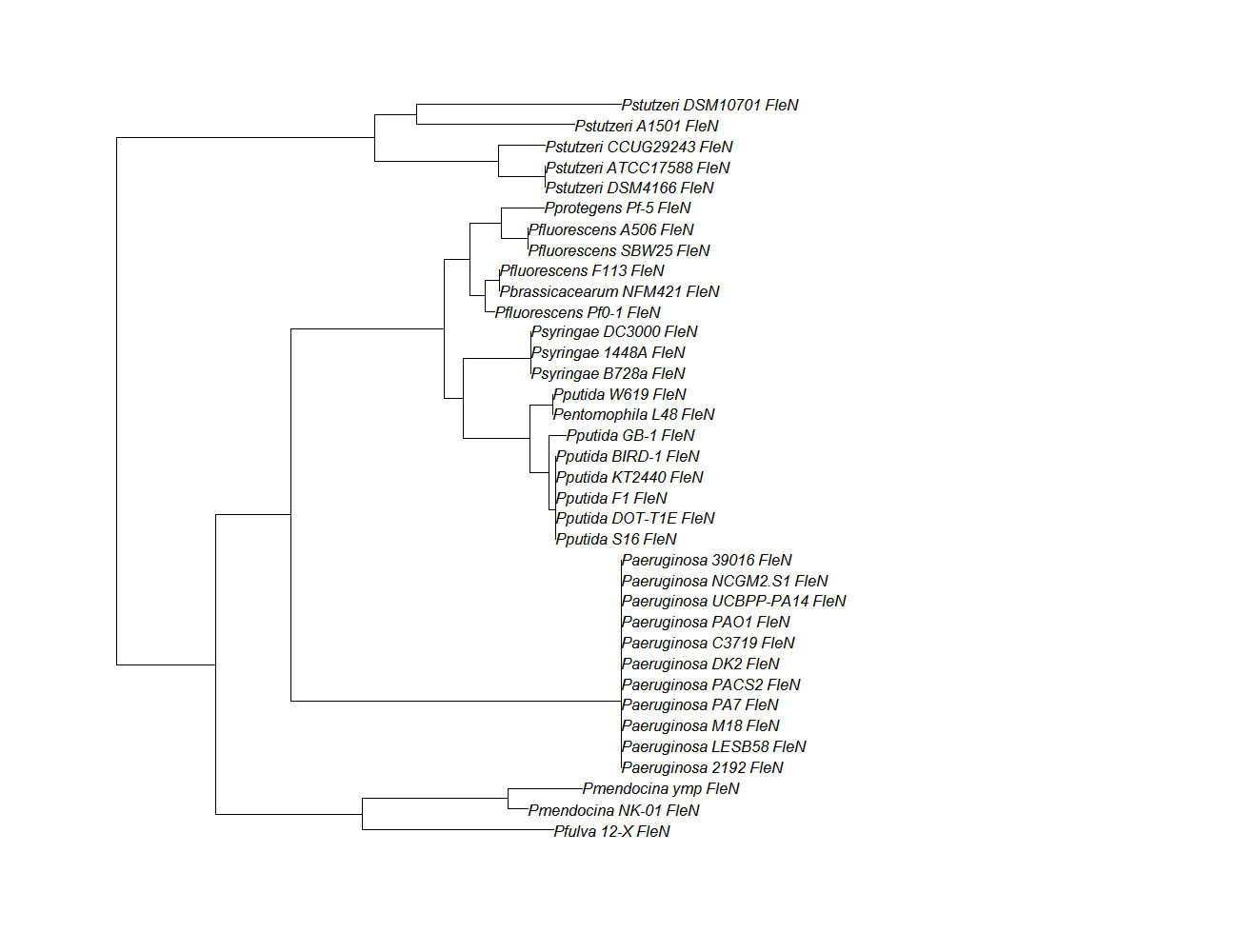 |
Conserved Domain |
| fleQ | fleQ pep alignment | 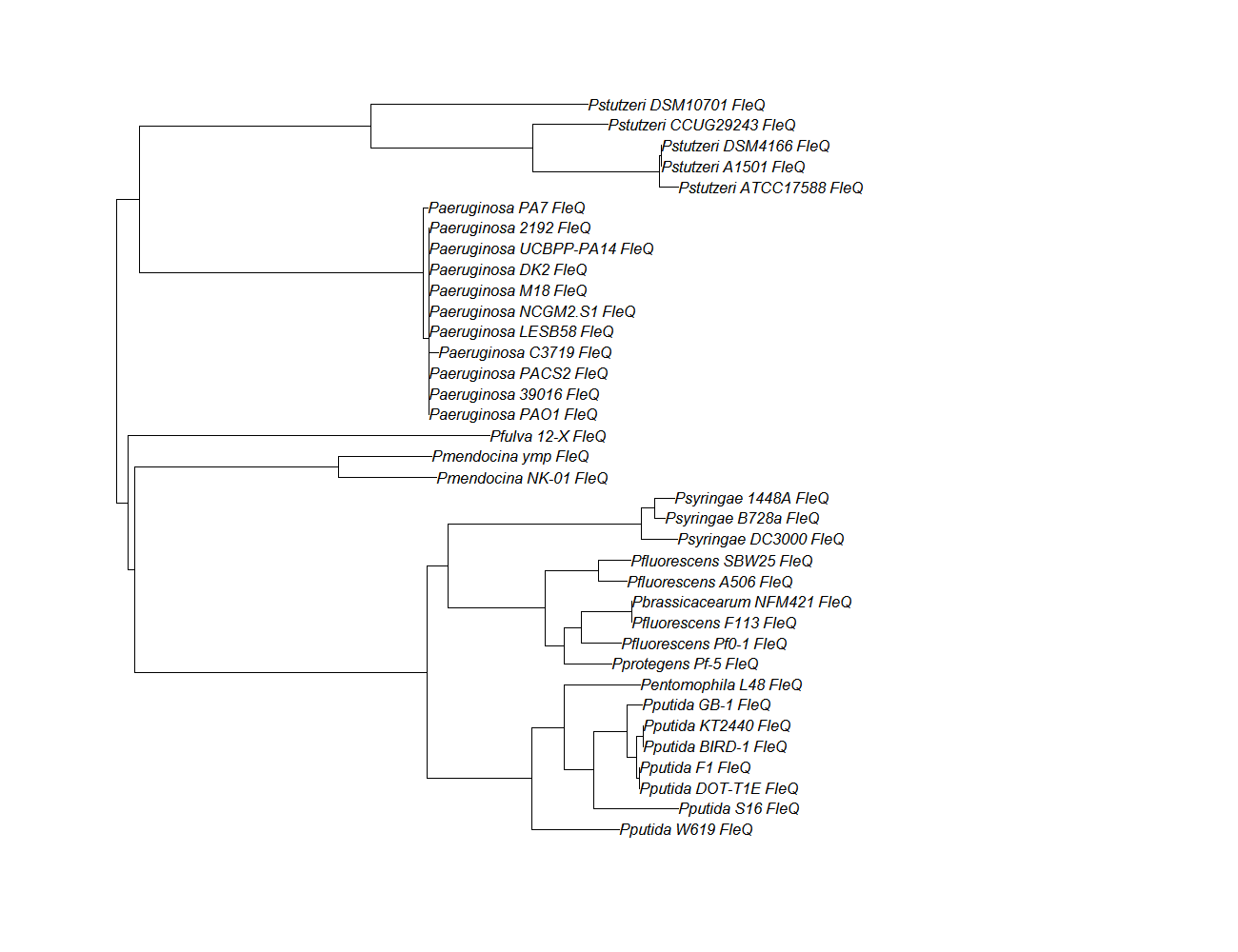 |
Conserved Domain |
| flhf | flhF pep alignment | 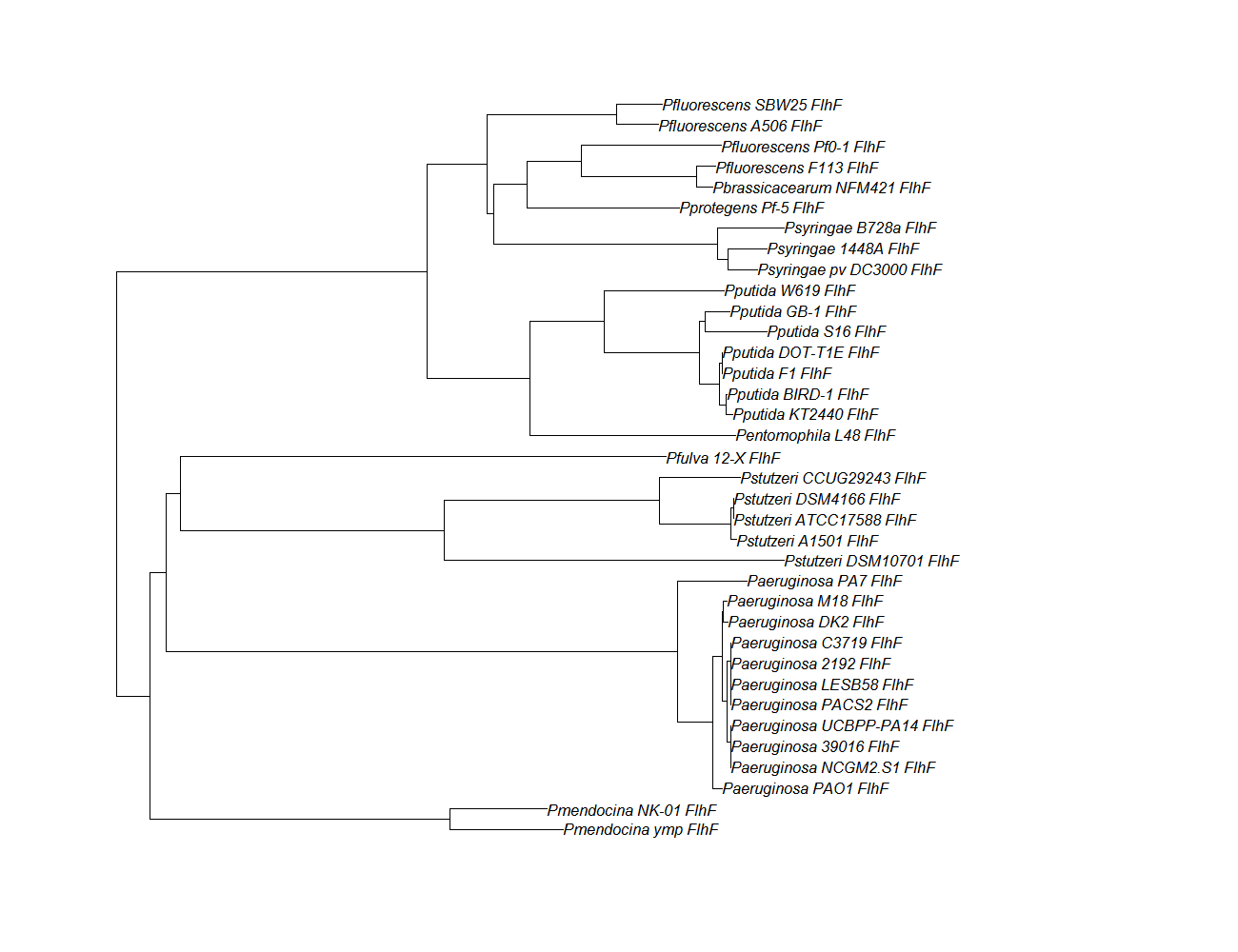 |
Conserved Domain |
Benchmark: June 11, 2013
- Finish parsing the genome files to upload the "orf" table (Raymond & Rayees)
- Rayees Parsed genome files: https://www.dropbox.com/sh/k0zktvvmv39op9i/1zBercEky8
- Parsing the ortholog file to upload the "orth_orf" table (Raymond)
- Identify and download fleN, fleQ, and flhF orthologs & align them (Rayees)