QuBi/modules/biol302: Difference between revisions
imported>Ppagan No edit summary |
imported>Ppagan No edit summary |
||
| Line 77: | Line 77: | ||
===[[QuBi/modules/biol302#Exercise2|Exercise]]=== | ===[[QuBi/modules/biol302#Exercise2|Exercise]]=== | ||
You're working on a research project. So far you've cloning this fragment: | You're working on a research project. So far you've cloning this fragment: | ||
<div style="font-family:Monospace;line-height:1;width: | <div style="font-family:Monospace;line-height:1;width:550px;border-style:solid;border-width:1px;border-color:#AAAAFF;background-color:#EEEEFF;padding-left:5px;padding-right:5px;padding-top:0px;padding-bottom:0px;"> | ||
TCTAGATGCATTTACGAAGGAGACAGAAAACGTCTTTCGGCAATAGCTCTCAAATGCAAAACGACGTCGG | |||
CGAGCTGTCCCTTACCTGGAGGCCCGCAGGAGAAGCGCGGTGATCCGAGAGGGTCCCCCAGGGGTGTCCG | |||
GTCGGTCTCCCGCTCGCCCAGCAGACGGCTGCGGAAACGGGGCAGCGTTTAAATAACCCCAGCTGGAGAC | |||
ATGTCAGGACTTAGCTCCTCCGACAGCCGACGCCGGACGTGTCCCAACTTGACCAGCCCCACAGGAAGAG | |||
CTGAGTCAACTCGGCCCAGCCCAGTCCCACCCGTCCCGGAAGCCGCATCCCGGCGAGTCCGGGACCAGGC | |||
ACCTGTCACCTCCTGGACCCCAGCAACGAGCCCAGCGCGACCCCGGAGCGGGCCCGAATTCTCTAGA | |||
</div> | </div> | ||
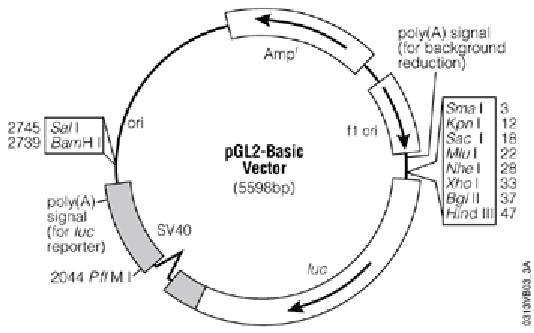
Into this [[vector]]: | Into this [[vector]]: | ||
Revision as of 23:05, 21 February 2013
- BIOL 302 Lab (Bioinformatics Exercises)
Research in molecular genetics requires effective use of bioinformatic tools to analyze and understand the genetic materials being worked with. The following exercises will expose you to real-world scenarios and introduce you to the methods and tools you can use to solve these problems.
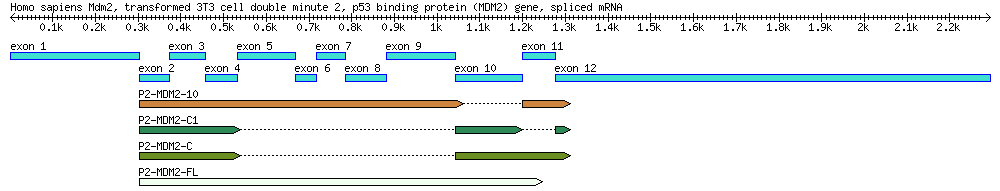
MODULE: Identification of mdm2 Splice Variants Using BLAST
A diagram of the MDM2 gene used in this exercise, along with its splice variants. By the end of this module you will create a similar diagram.
Objectives
- Learn to use Genbank database and BLAST tool to analyze nucleotide sequences
- Use BLAST to identify
Key Concepts
- Blast
- Genbank
- Annotation
- Accession Number
- Alternative Splicing
Exercise
| Genbank Accession # | cDNA Clone | Description | Cell Line | Length (bp) |
|---|---|---|---|---|
| AF527840 | Genomic DNA | 34,088 | ||
| EU076746 | P2-MDM2-C1 | cDNA missing exons 5-9 & 11 | MANCA | 427 |
| EU076747 | P2-MDM2-10 | cDNA missing exon 10 | ML-1 | 842 |
| EU076748 | P2-MDM2-C | cDNA missing exons 5-9 | A876 | 505 |
| EU076749 | P2-MDM2-FL | Full-length cDNA | SJSA-1 | 845 |
- Explore the gene annotation for AF527840.
Sequences on genbank have both basic reference information (such as what the sequence is, what organism it came from, and bibliographical information) and sequence annotations. Some sequences are more richly annotated than others - it is up to researchers to annotate the sequences they generate, which requires extra work. For this exercise you will be working will a well-annotated sequence: accession number AF527840. Explore its annotation and use it to complete the following set of tasks.
- DRAW a diagram of this gene using the information and coordinates listed in the annotation. (Note: this is the bulk of the assignment and this diagram is needed for the last set of questions. Don't get lazy on this.)
- Label the top of the diagram with basic information, such as the gene's name, organism, etc.. Someone should be able to pick up your diagram and know exactly what they're looking at.
- Including introns, exons, 3'/5' UTRs, +1, and exact coordinates. (The mRNA annotation states which segments are used to create mRNA, and the CDS annotation states which parts code amino acids (CDS = coding sequence)).
- Draw the diagram mostly to scale. It does NOT have to be perfect, but make a reasonable effort. Put a scale bar and length markers on your drawing.
- How does the sequence vary at positions X, X, and X for this gene? Do these change the AA for the resulting peptide?
- What kinds of repeat regions can be found in this gene?
- Explore the graphical presentation of the gene to answer the following question.
Genbank provides graphical representations of the sequences on its database: click the "Graphics" link below the sequence title, OR click "Display Settings" above the title, and choose "Graphics". Take a few minutes to explore this graphical browser and answer the following question:
- A question that can only be answered from looking at this graph.
- Another question that can only be answered from looking at this graph.
- Use BLAST to determine which exons are used in the mRNA transcripts.
This is the most "bioinformatic" part of the assignment. Blast ALL FOUR of the mRNA sequences (EU076746, EU076747, EU076748, EU076749) against the main sequence (AF527840) and use the results to answer the following questions. If you labeled your diagram well (with coordinates!), this task should go by quickly.
- Which exons are used to create EU076746, EU076747, EU076748, EU076749?
- Do the BLAST search results corresponding to exons exactly match the start/end positions of the exons as labeled in your diagram? If not, what is the most likely reason for this?
- Do any of the BLAST results match regions outside of exons? If so, what regions?
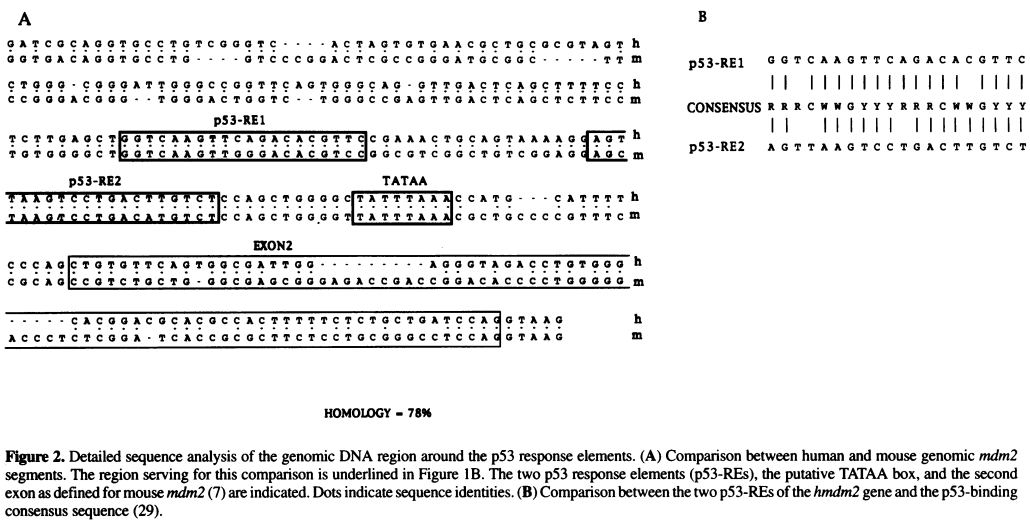
MODULE: Cloning of murine mdm2 gene sequence to study cis acting DNA elements
Key Concepts
- Clone
- Vector
- Rotational Symmetry
Exercise
You're working on a research project. So far you've cloning this fragment:
TCTAGATGCATTTACGAAGGAGACAGAAAACGTCTTTCGGCAATAGCTCTCAAATGCAAAACGACGTCGG CGAGCTGTCCCTTACCTGGAGGCCCGCAGGAGAAGCGCGGTGATCCGAGAGGGTCCCCCAGGGGTGTCCG GTCGGTCTCCCGCTCGCCCAGCAGACGGCTGCGGAAACGGGGCAGCGTTTAAATAACCCCAGCTGGAGAC ATGTCAGGACTTAGCTCCTCCGACAGCCGACGCCGGACGTGTCCCAACTTGACCAGCCCCACAGGAAGAG CTGAGTCAACTCGGCCCAGCCCAGTCCCACCCGTCCCGGAAGCCGCATCCCGGCGAGTCCGGGACCAGGC ACCTGTCACCTCCTGGACCCCAGCAACGAGCCCAGCGCGACCCCGGAGCGGGCCCGAATTCTCTAGA
Into this vector:
Using these restriction enzymes:
| Restriction Enzyme | Recognition Sequence | Overhang |
|---|---|---|
| XbaI | TCTAGA | CTAG |
| NheI | GCTAGC | CTAG |
In this module, you will analyze your fragment sequence and resultant clone with the help of this diagram:
PROPERLY CITE THIS Taken from A functional p53-responsive intronic promoter is contained within the human mdm2 gene. Pubmed PDF
- Annotate fragment and draw diagram of resultant clone
This exercise is not bioinformatics-heavy, but sequence analysis is a major task of the field. You will create another annotated drawing of a DNA molecule (the plasmid with your fragment cloned in) and annotate your fragment sequence by hand. You should have a printout of the fragment sequence to directly annotate.
- On your sequence,