Summer 2018: Difference between revisions
imported>Lab No edit summary |
imported>Lab |
||
| Line 262: | Line 262: | ||
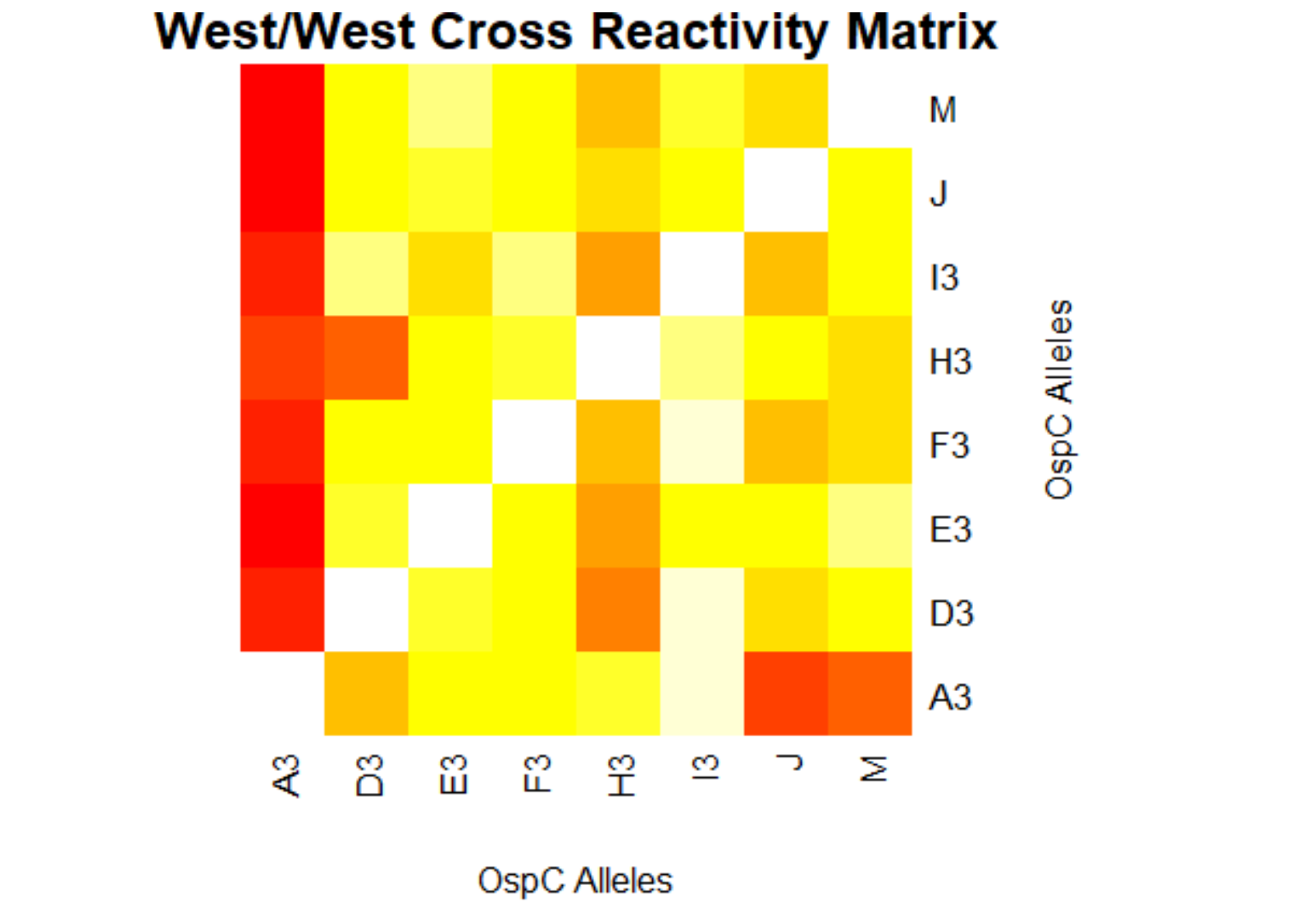
[[File:westwest.png|800px|thumb|left|West/West Cross Reactivity Matrix]] | [[File:westwest.png|800px|thumb|left|West/West Cross Reactivity Matrix]] | ||
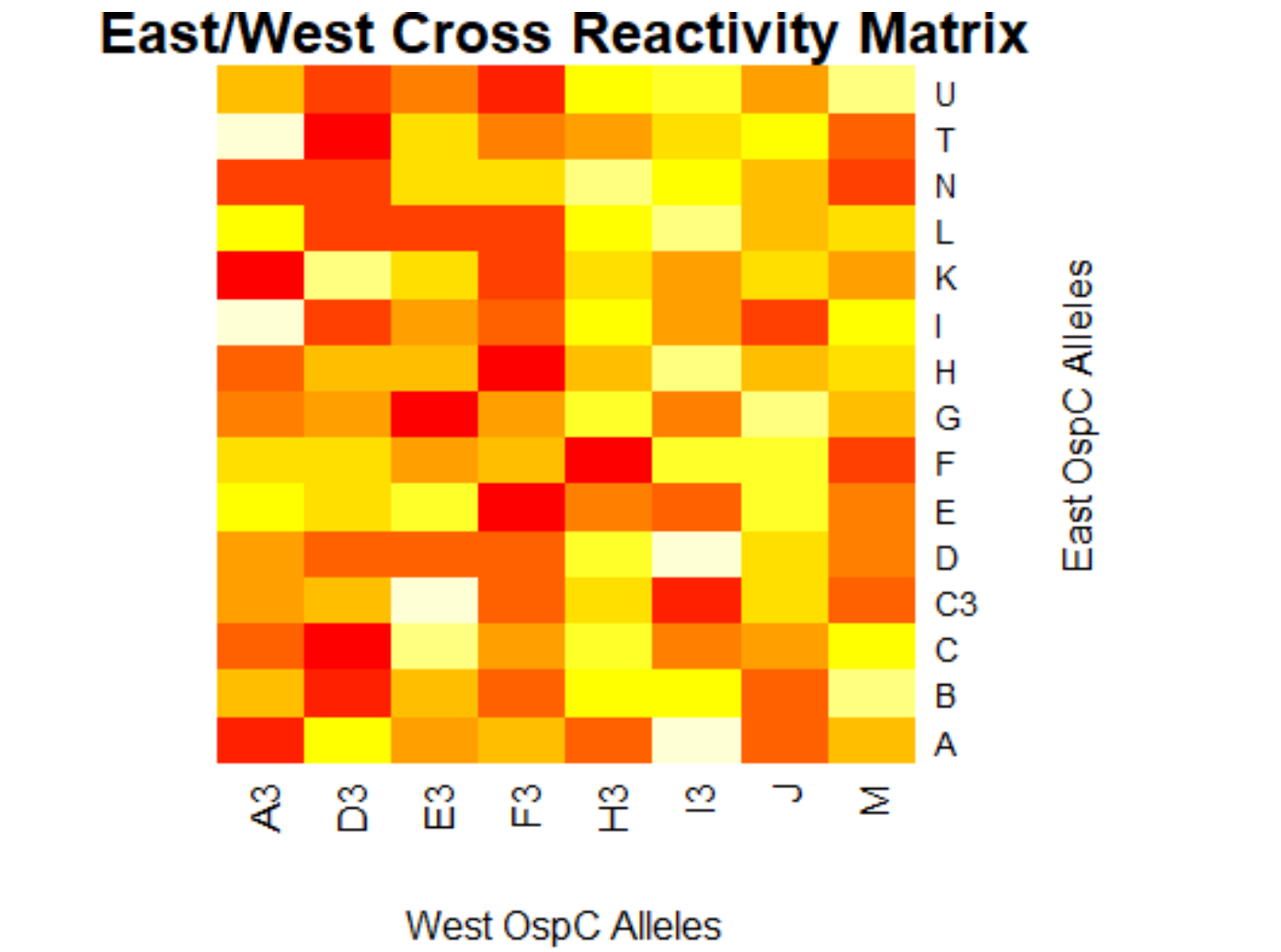
[[File:eastwest.png|800px|thumb|left|West/West Cross Reactivity Matrix]] | |||
Revision as of 20:29, 19 July 2018
Rules of Conduct
- No eating, drinking, or loud talking in the lab. Socialize in the lobby only.
- Be respectful to each other, regardless of level of study
- Be on time & responsible. Communicate in advance with the PI if late or absent
Participants
- Dr Oliver Attie, Research Associate
- Brian Sulkow, Research Associate
- Saymon Akther, CUNY Graduate Center, EEB Program
- Lily Li, CUNY Graduate Center, EEB Program
- Mei Wu, Bioinformatics Research Assistant
- Yinheng Li, Informatics Research Assistant
- Christopher Panlasigui, Hunter Biology
- Dr Lia Di, Senior Scientist
- Dr Weigang Qiu, Principal Investigator
- Summer Interns: Muhammad, Pavan, Roman, Benjamin, Andrew, Michelle, Hannah
Journal Club
- a Unix & Perl tutorial
- A short introduction to molecular phylogenetics: http://www.ncbi.nlm.nih.gov/pubmed/12801728
- A review on Borrelia genomics: https://www.ncbi.nlm.nih.gov/pubmed/24704760
- ospC epitope mapping: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0067445
- Codon usage changes fitness in E.coli: Frumkin et al (2018) "Codon usage of highly expressed genes affects proteome-wide translation efficiency". PNAS
Projects
Borrelia genome evolution (Led by Saymon)
- Goal 1. Estimate time of cross-Atlantic dispersal using core-genome sequences
- Goal 2. Investigate codon biases with respect to levels of gene expression. Data file:
* Andrew's BioPython code to calculate CAI
#Opens the fasta file and reads contents into a string (myStr).
myFile = open("B31-cp26.txt","r")
myStr = myFile.read()
myFile.close()
#Imports codon usage module.
from Bio.SeqUtils import CodonUsage as cu
#Takes myStr and processes it into a list of sequences (FastaList).
FastaList = myStr.split(">")
FastaList = FastaList[1:]
IDList = []
EnterList = []
##Separates FastaList into a list of sequence IDs (IDList) and a list of sequences (EnterList).
for seq in FastaList:
IDList += [seq[:6]]
EnterList += [seq[6:]]
##Removes enter characters from each sequence in EnterList.
SeqList = []
for seq in EnterList:
SeqStr = seq.replace("\n", "")
SeqList += [SeqStr]
#Calculates and presents the CAI value for each sequence using functions from the module.
myObject = cu.CodonAdaptationIndex()
myObject.generate_index("B31-cp26.txt")
for SeqIndex in range(len(SeqList)):
print (IDList[SeqIndex], ' CAI =', myObject.cai_for_gene(SeqList[SeqIndex]))
Output for cp26:
BB_B01 CAI = 0.7190039074113422
BB_B02 CAI = 0.678404951527374
BB_B03 CAI = 0.6893076488255271
BB_B04 CAI = 0.7250154635421513
BB_B05 CAI = 0.6971190458423587
BB_B06 CAI = 0.67042305582205
BB_B07 CAI = 0.6971020959083346
BB_B09 CAI = 0.6786931743972611
BB_B10 CAI = 0.7224886929887183
BB_B12 CAI = 0.6997502136447451
BB_B13 CAI = 0.7592966148479222
BB_B14 CAI = 0.6959525612884284
BB_B16 CAI = 0.6835709626613392
BB_B17 CAI = 0.6974779110749645
BB_B18 CAI = 0.7052250722958308
BB_B19 CAI = 0.7049049245887261
BB_B22 CAI = 0.6860641572293008
BB_B23 CAI = 0.6915165725213809
BB_B24 CAI = 0.7025276490965267
BB_B25 CAI = 0.7439914547011712
BB_B26 CAI = 0.7255623088410704
BB_B27 CAI = 0.7161378416520467
BB_B28 CAI = 0.7316661839512337
BB_B29 CAI = 0.6919705705489939
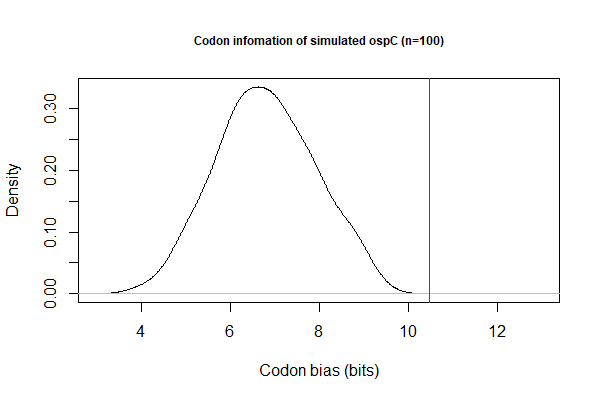
- Codon bias by Shannon information: BASH pipeline
- Simulate 100 sequences for each CDS:
cat filename.txt | while read line; do ./codon-info-sim.pl -n 100 BbB31.cutg.GCG "$line".fas > "$line"-sim.fas; done; - Calculate Shannon index for each CDS (simulated & actual)
./codon-info.pl BbB31.cutg.GCG foo.fas
- Simulate 100 sequences for each CDS:
Identification of host species from ticks (Led by Lily [after first-level])
- Goal 1. Protocol optimization for PCR amplification of host DNA from ticks
- Goal 2. Protocol development: library construction for MiSeq
- Goal 3. Development of bioinformatics protocols and sequence database
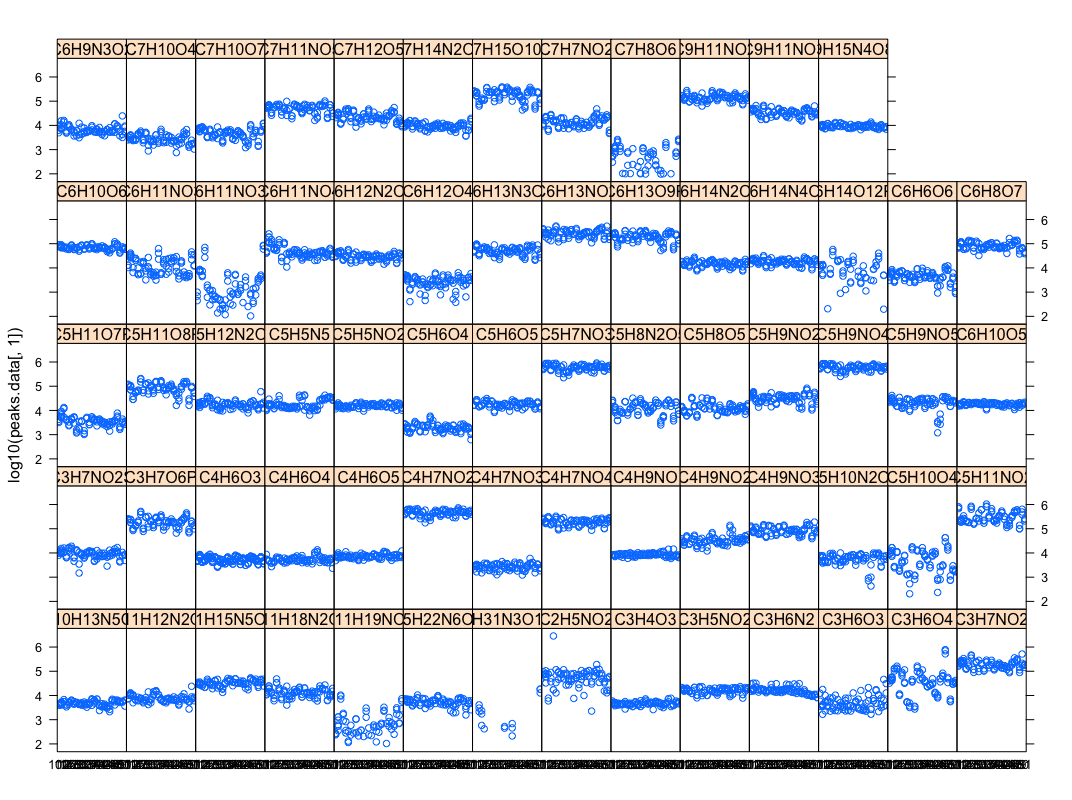
Pseudomonas Genome-wide Association Studies (GWAS) (Led by Mai & Yinheng, in collaboration with Dr Xavier of MSKCC)
- Goal 1. Association of genes/SNPs with biofilm formation and c-di-GMP levels: Manuscript preparation
- Goal 2. Association of genome diversity with metabolic diversity
- (Christopher) This script parses excel peak-area file into database & R inputs
#!/usr/bin/perl -w
use strict;
use Data::Dumper;
use Getopt::Std;
my %opts;
my $line_ct = 0;
my (@colnames, @areas, %seen_cmps, %seen_gids);
getopts('dr', \%opts);
while(<>) {
chomp;
$line_ct++;
next unless $line_ct >=4;
if ($line_ct == 4) {
@colnames = split "\t", $_;
for (my $i=5; $i<=$#colnames; $i++) { $seen_gids{$colnames[$i]}++ } # get uniq gids
next;
}
my @data = split "\t", $_;
$seen_cmps{$data[1]}++; # get unique compound formula
for (my $i=5; $i<=$#data; $i++) {
my $area = { 'compound' => $data[1], 'gid' =>$colnames[$i], 'peak_area' => $data[$i]};
push @areas, $area;
}
}
if ($opts{d}) { # for database output
foreach my $cmp (sort keys %seen_cmps) {
foreach my $gid (sort keys %seen_gids) {
my @peaks = grep { $_->{compound} eq $cmp && $_->{gid} == $gid } @areas;
my $peak_str = join ",", map {$_->{peak_area} || "NULL"} @peaks;
print join "\t", ($gid, $cmp, "{" . $peak_str . "}");
print "\n";
}
}
}
if ($opts{r}) { # for R output
foreach my $cmp (sort keys %seen_cmps) {
foreach my $gid (sort keys %seen_gids) {
my @peaks = grep { $_->{compound} eq $cmp && $_->{gid} == $gid } @areas;
foreach my $peak (@peaks) {
next unless $peak->{peak_area};
print join "\t", ($peak->{peak_area}, $gid, $cmp);
print "\n";
}
}
}
}
exit;
Machine learning approaches to evolution (Led by Oliver & Brian)
- Goal 1. Implement Hopfield network for optimization of protein structure
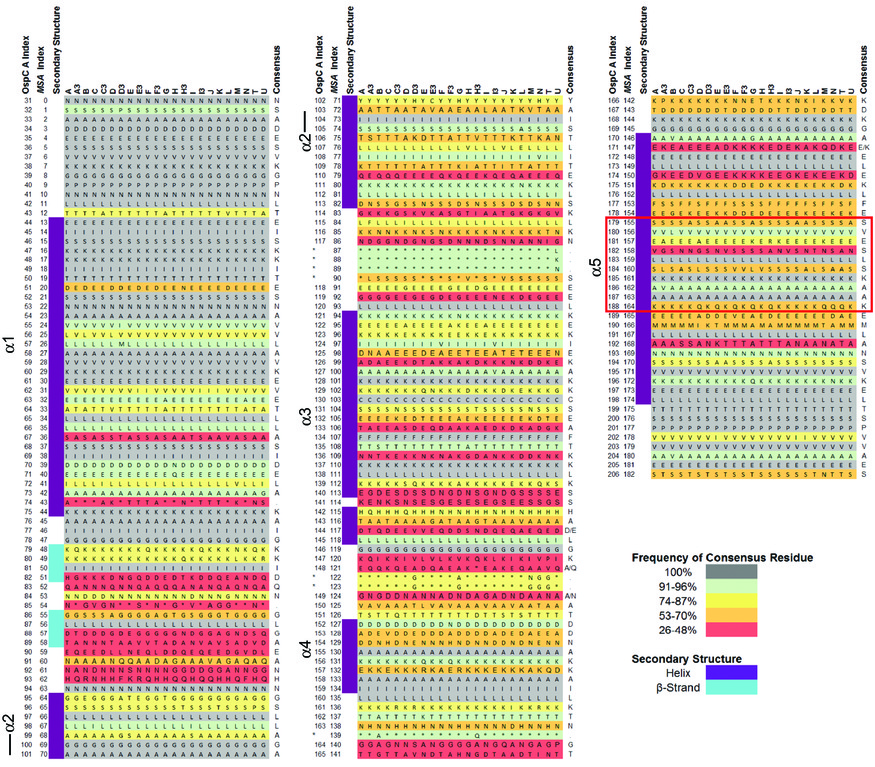
- Goal 2. Neural-net models of OspC. Structural alignment (S2 from Baum et al 2013):
- Goal 3. K-mer-based pipeline for genome classification
Weekly Schedule
- Summer kickoff (June 1, 2018, Friday): Introduction & schedules
- Week 1 (June 4-8):
- Monday: the Unix & Perl Tutorial, Part 1
- Tuesday: Unix Part2. Explore the "iris" data set using R, by following the the Monte Carlo Club Week 1 (1 & 2) and Week 2. Read McKay (2003), Chapters 38 & 39
- Thursday: 1st field day (Caumsett State Park); Participants: John, Muhammad, Pavan, Andrew, Dr Sun, Weigang, with 3 members of Moses team from Suffolk County Vector Control. Got ~110 deer tick nymphs
- Friday: meeting with MSKCC group at 11am; BBQ afterwards
- Week 2 (June 11-15):
- Monday: Lab meeting, projects assigned
- Tuesday: neural net tutorial (by Brian)
- Thursday: 2nd field day (Fire Island National Seashore). Participants: John, Brian, Mei, Muhammad, Pavan, Benjamin, and Weigang. Got ~100 lone-star ticks and 4 deer tick nymphs
- Week 3 (June 18-22):
- Monday: Lab meeting, 1st project reports
- Codon Bias: Theory, Coding, and Data (Andrew, Pavan, Saymon)
- OspC epitope identification: Serum correlation, sequence correlation, immunity-sequence correction (Muhammad, Roman, Brian)
- Pseudomonas metabolomics: parsing intensity file; theory & parsing SMBL file (Chris & Benjamin)
- Tuesday: working groups
- Wed: working groups
- Thursday: Big Data Workshop
- Friday: working groups
- Monday: Lab meeting, 1st project reports
- Week 4 (June 25-29):
- Monday: Lab meeting
- Tu-Th: work sessions
- Friday: Joint lab meeting with MSKCC/Lab visit
- Week 5 (July 2-6):
- Monday: Lab meeting
- Tuesday/Wed: 4th of July Break
- Thursday & Friday: work sessions
- Week 6 (July 9-13)
- Monday (July 9): E-reports & Wiki posts
- Week 7 (July 16-20)
- Monday (July 16): E-reports * Wiki posts
- Week 8 (July 23-27)
- Monday: lab meeting resumes
Lab notes for Summer HS Interns
- NCI Cloud: Seven Bridges Cloud Platform. Create an user account
- Read documentation & tutorials: Documentation
Notes & Scripts
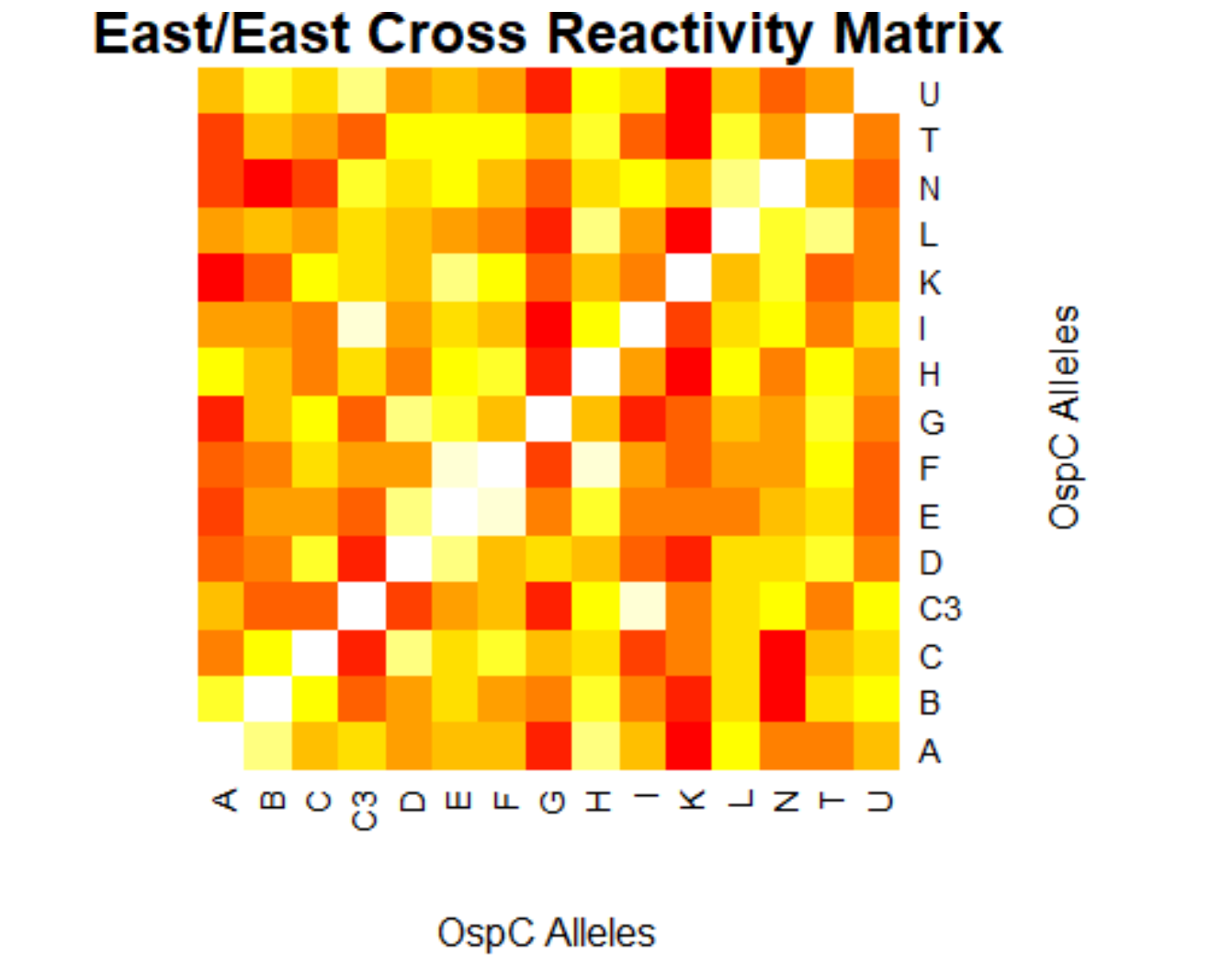
- (Weigang) A sample R script to parse Table S2 from Baum et al 2013, sera-antigen reactivity measurements
# preliminaries: save as TSV; substitute "\r" if necessary;
# substitute "N/A" to "NA"; remove extra columns
setwd("Downloads/")
x <- read.table("table-s2.txt4", sep="\t", header=T)
View(x)
colnames(x)
which(x[,8]=="A")
x[which(x[,8]=="A"),12]
x[which(x[,8]=="A3"),12]
cor.test(x[which(x[,8]=="A3"),12], x[which(x[,8]=="A"),12], method = "pearson")
x.cor$estimate
levels(x[,8]) # obtain ospC allele types; to be looped through in pairwise
for (i in 1:?) { for (j in ?:?) {cor.test(....)}}
- (Muhammad) Output generates data frame of correlation/p values for 23 different Osp-C allele types in pairwise
setwd("C:/R_OspC")
x <- read.table("Table-S2.txt", sep="\t", header=T)
a<-levels(x[,8])
output = data.frame(i=character(), j=character(), cor = numeric(), p = numeric());
#k <-0;
for(i in 1:22) {
allele.i <- a[i];
vect.i <- x[which(x[,8]==allele.i),12];
for(j in (i+1):23) {
allele.j <- a[j];
vect.j <-x[which(x[,8]==allele.j),12];
cor <- cor.test(vect.i,vect.j, method = "pearson");
output <- rbind (output, data.frame(i=allele.i, j=allele.j, cor=cor$estimate, p=cor$p.value));
}
}
write.table(output, "immune-output.txt", quote = F, sep = "\t")
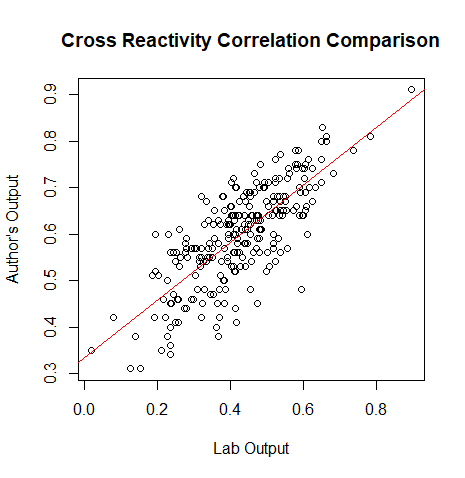
- (Muhammad) Creates a plot for the correlation values of the lab's data and the author's data
#read in the authors cross reactivity correlation matrix
cr<- read.csv("C:/ospc/matricesospc.csv", header=F, sep = ",")
#puts all of the values of cr into corvect
corvect<-c()
for (i in 1:(nrow(cr)-1)) {
for (j in (i+1):ncol(cr)) {
corvect[length(corvect)+1]<- cr[i,j]
}
}
#merging cross reactivity correlation data and the authors data
df<- data.frame(output, corvect)
#plots the dataframe
plot(output[,3], corvect, main="Cross Reactivity Correlation Comparison", ylab = "Author's Output", xlab="Lab Output")
#gives the liner model, relationship between our data and the authors
b<-lm(corvect~output[,3])
#places the ab line on the plot
abline(b, col=2)